Understanding Clinical Trial Design - Research Advocacy Network
Understanding Clinical Trial Design - Research Advocacy Network
Understanding Clinical Trial Design - Research Advocacy Network
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
24<br />
UNDERSTANDING CLINICAL TRIAL DESIGN: A TUTORIAL FOR RESEARCH ADVOCATES<br />
The overall conceptual model of the scientific method (Figure 4) holds for both<br />
Bayesians and frequentists. Likewise, the issues of randomization and blinding<br />
hold for both approaches. However, the Bayesian approach provides an alternative<br />
process for carrying out the inferential steps that allow researchers to draw<br />
conclusions about populations of patients, based on samples in their trials. The<br />
Bayesian inferential process will be described below, and contrasted to the process<br />
employed by frequentists that was described in the previous section. Although the<br />
frequentist approach has been widely used, the logic is quite contorted. Further,<br />
progress has been slow because trials are large and expensive.<br />
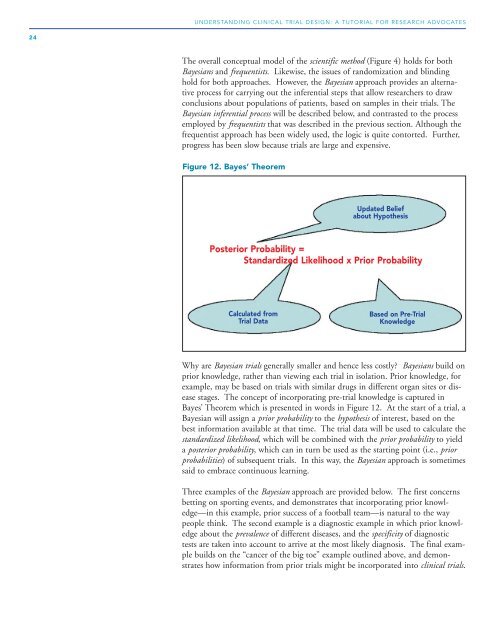
Figure 12. Bayes’ Theorem<br />
Updated Belief<br />
about Hypothesis<br />
Posterior Probability =<br />
Standardized Likelihood x Prior Probability<br />
Calculated from<br />
<strong>Trial</strong> Data<br />
Based on Pre-<strong>Trial</strong><br />
Knowledge<br />
Why are Bayesian trials generally smaller and hence less costly? Bayesians build on<br />
prior knowledge, rather than viewing each trial in isolation. Prior knowledge, for<br />
example, may be based on trials with similar drugs in different organ sites or disease<br />
stages. The concept of incorporating pre-trial knowledge is captured in<br />
Bayes’ Theorem which is presented in words in Figure 12. At the start of a trial, a<br />
Bayesian will assign a prior probability to the hypothesis of interest, based on the<br />
best information available at that time. The trial data will be used to calculate the<br />
standardized likelihood, which will be combined with the prior probability to yield<br />
a posterior probability, which can in turn be used as the starting point (i.e., prior<br />
probabilities) of subsequent trials. In this way, the Bayesian approach is sometimes<br />
said to embrace continuous learning.<br />
Three examples of the Bayesian approach are provided below. The first concerns<br />
betting on sporting events, and demonstrates that incorporating prior knowledge—in<br />
this example, prior success of a football team—is natural to the way<br />
people think. The second example is a diagnostic example in which prior knowledge<br />
about the prevalence of different diseases, and the specificity of diagnostic<br />
tests are taken into account to arrive at the most likely diagnosis. The final example<br />
builds on the “cancer of the big toe” example outlined above, and demonstrates<br />
how information from prior trials might be incorporated into clinical trials.