Understanding Clinical Trial Design - Research Advocacy Network
Understanding Clinical Trial Design - Research Advocacy Network
Understanding Clinical Trial Design - Research Advocacy Network
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
UNDERSTANDING CLINICAL TRIAL DESIGN: A TUTORIAL FOR RESEARCH ADVOCATES<br />
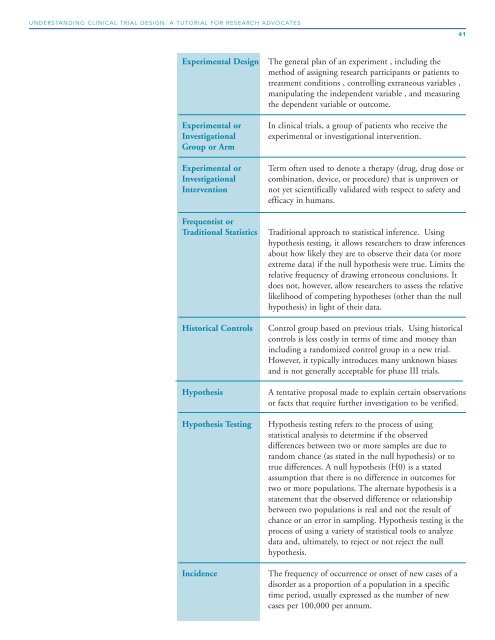
Experimental <strong>Design</strong> The general plan of an experiment , including the<br />
method of assigning research participants or patients to<br />
treatment conditions , controlling extraneous variables ,<br />
manipulating the independent variable , and measuring<br />
the dependent variable or outcome.<br />
Experimental or In clinical trials, a group of patients who receive the<br />
Investigational experimental or investigational intervention.<br />
Group or Arm<br />
Experimental or Term often used to denote a therapy (drug, drug dose or<br />
Investigational combination, device, or procedure) that is unproven or<br />
Intervention not yet scientifically validated with respect to safety and<br />
efficacy in humans.<br />
Frequentist or<br />
Traditional Statistics Traditional approach to statistical inference. Using<br />
hypothesis testing, it allows researchers to draw inferences<br />
about how likely they are to observe their data (or more<br />
extreme data) if the null hypothesis were true. Limits the<br />
relative frequency of drawing erroneous conclusions. It<br />
does not, however, allow researchers to assess the relative<br />
likelihood of competing hypotheses (other than the null<br />
hypothesis) in light of their data.<br />
Historical Controls Control group based on previous trials. Using historical<br />
controls is less costly in terms of time and money than<br />
including a randomized control group in a new trial.<br />
However, it typically introduces many unknown biases<br />
and is not generally acceptable for phase III trials.<br />
Hypothesis A tentative proposal made to explain certain observations<br />
or facts that require further investigation to be verified.<br />
Hypothesis Testing Hypothesis testing refers to the process of using<br />
statistical analysis to determine if the observed<br />
differences between two or more samples are due to<br />
random chance (as stated in the null hypothesis) or to<br />
true differences. A null hypothesis (H0) is a stated<br />
assumption that there is no difference in outcomes for<br />
two or more populations. The alternate hypothesis is a<br />
statement that the observed difference or relationship<br />
between two populations is real and not the result of<br />
chance or an error in sampling. Hypothesis testing is the<br />
process of using a variety of statistical tools to analyze<br />
data and, ultimately, to reject or not reject the null<br />
hypothesis.<br />
Incidence The frequency of occurrence or onset of new cases of a<br />
disorder as a proportion of a population in a specific<br />
time period, usually expressed as the number of new<br />
cases per 100,000 per annum.<br />
41