Understanding Clinical Trial Design - Research Advocacy Network
Understanding Clinical Trial Design - Research Advocacy Network
Understanding Clinical Trial Design - Research Advocacy Network
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
UNDERSTANDING CLINICAL TRIAL DESIGN: A TUTORIAL FOR RESEARCH ADVOCATES<br />
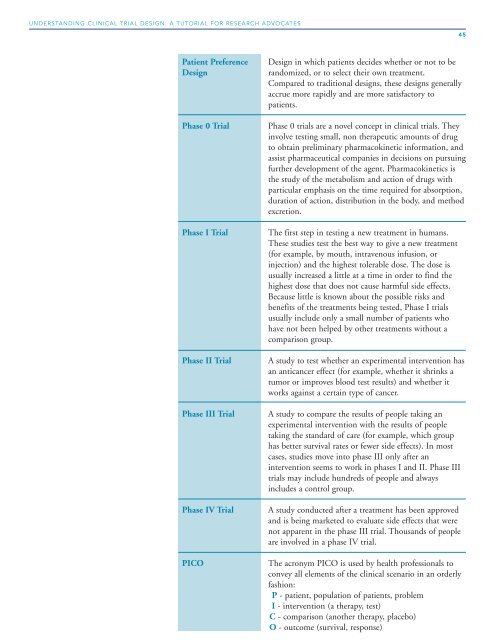
Patient Preference <strong>Design</strong> in which patients decides whether or not to be<br />
<strong>Design</strong> randomized, or to select their own treatment.<br />
Compared to traditional designs, these designs generally<br />
accrue more rapidly and are more satisfactory to<br />
patients.<br />
Phase 0 <strong>Trial</strong> Phase 0 trials are a novel concept in clinical trials. They<br />
involve testing small, non therapeutic amounts of drug<br />
to obtain preliminary pharmacokinetic information, and<br />
assist pharmaceutical companies in decisions on pursuing<br />
further development of the agent. Pharmacokinetics is<br />
the study of the metabolism and action of drugs with<br />
particular emphasis on the time required for absorption,<br />
duration of action, distribution in the body, and method<br />
excretion.<br />
Phase I <strong>Trial</strong> The first step in testing a new treatment in humans.<br />
These studies test the best way to give a new treatment<br />
(for example, by mouth, intravenous infusion, or<br />
injection) and the highest tolerable dose. The dose is<br />
usually increased a little at a time in order to find the<br />
highest dose that does not cause harmful side effects.<br />
Because little is known about the possible risks and<br />
benefits of the treatments being tested, Phase I trials<br />
usually include only a small number of patients who<br />
have not been helped by other treatments without a<br />
comparison group.<br />
Phase II <strong>Trial</strong> A study to test whether an experimental intervention has<br />
an anticancer effect (for example, whether it shrinks a<br />
tumor or improves blood test results) and whether it<br />
works against a certain type of cancer.<br />
Phase III <strong>Trial</strong> A study to compare the results of people taking an<br />
experimental intervention with the results of people<br />
taking the standard of care (for example, which group<br />
has better survival rates or fewer side effects). In most<br />
cases, studies move into phase III only after an<br />
intervention seems to work in phases I and II. Phase III<br />
trials may include hundreds of people and always<br />
includes a control group.<br />
Phase IV <strong>Trial</strong> A study conducted after a treatment has been approved<br />
and is being marketed to evaluate side effects that were<br />
not apparent in the phase III trial. Thousands of people<br />
are involved in a phase IV trial.<br />
PICO The acronym PICO is used by health professionals to<br />
convey all elements of the clinical scenario in an orderly<br />
fashion:<br />
P - patient, population of patients, problem<br />
I - intervention (a therapy, test)<br />
C - comparison (another therapy, placebo)<br />
O - outcome (survival, response)<br />
45