Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
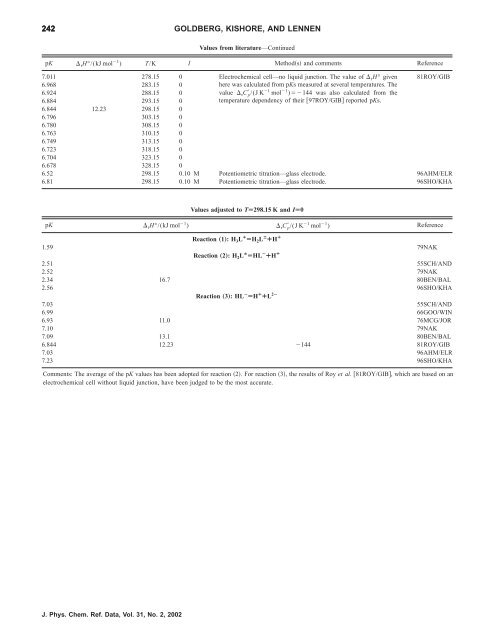
242 GOLDBERG, KISHORE, AND LENNEN<br />
Values from literature—Continued<br />
pK rH°/(kJ mol 1 ) T/K I Methods and comments Reference<br />
7.011 278.15 0 Electrochemical cell—no liquid junction. The value <strong>of</strong> rH° given<br />
6.968 283.15 0<br />
6.924 288.15 0<br />
6.884 293.15 0<br />
6.844 12.23 298.15 0<br />
6.796 303.15 0<br />
6.780 308.15 0<br />
6.763 310.15 0<br />
6.749 313.15 0<br />
6.723 318.15 0<br />
6.704 323.15 0<br />
6.678 328.15 0<br />
here was calculated from pKs measured at several temperatures. The<br />
value rC p /(J K 1 mol 1 )144 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 97ROY/GIB reported pKs.<br />
81ROY/GIB<br />
6.52 298.15 0.10 M Potentiometric titration—glass electrode. 96AHM/ELR<br />
6.81 298.15 0.10 M Potentiometric titration—glass electrode. 96SHO/KHA<br />
Values adjusted to TÄ298.15 K and IÄ0<br />
pK rH°/(kJ mol 1 ) rC p /(J K 1 mol 1 ) Reference<br />
Reaction „1…: H 3L ¿ ÄH 2L Á ¿H ¿<br />
1.59 79NAK<br />
Reaction „2…: H 2L Á ÄHL À ¿H ¿<br />
2.51 55SCH/AND<br />
2.52 79NAK<br />
2.34 16.7 80BEN/BAL<br />
2.56 96SHO/KHA<br />
Reaction „3…: HL À ÄH ¿ ¿L 2À<br />
7.03 55SCH/AND<br />
6.99 66GOO/WIN<br />
6.93 11.0 76MCG/JOR<br />
7.10 79NAK<br />
7.09 13.1 80BEN/BAL<br />
6.844 12.23 144 81ROY/GIB<br />
7.03 96AHM/ELR<br />
7.23 96SHO/KHA<br />
Comments: The average <strong>of</strong> <strong>the</strong> pK values has been adopted <strong>for</strong> reaction 2. For reaction 3, <strong>the</strong> results <strong>of</strong> Roy et al. 81ROY/GIB, which are based on an<br />
electrochemical cell without liquid junction, have been judged to be <strong>the</strong> most accurate.<br />
J. Phys. Chem. Ref. Data, Vol. 31, No. 2, 2002