Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
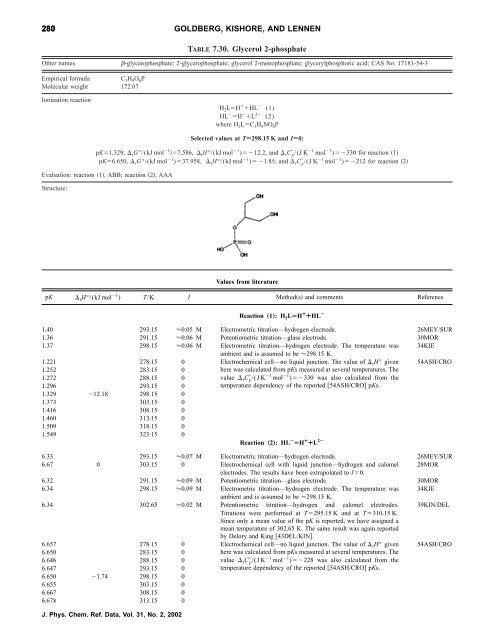
280 GOLDBERG, KISHORE, AND LENNEN<br />
TABLE 7.30. Glycerol 2-phosphate<br />
O<strong>the</strong>r names -glycerophosphate; 2-glycerophosphate; glycerol 2-monophosphate; glycerylphosphoric acid; CAS No. 17181-54-3<br />
Empirical <strong>for</strong>mula C 3H 9O 6P<br />
Molecular weight 172.07<br />
<strong>Ionization</strong> reaction<br />
H 2LH HL (1)<br />
HL H L 2 (2)<br />
where H 2LC 3H 9NO 6P<br />
Selected values at TÄ298.15 K and IÄ0:<br />
pK1.329, rG°/(kJ mol 1 7.586, rH°/(kJ mol 1 )12.2, and rC p /(J K 1 mol 1 )330 <strong>for</strong> reaction 1<br />
pK6.650, rG°/(kJ mol 1 )37.958, rH°/(kJ mol 1 )1.85, and rC p /(J K 1 mol 1 )212 <strong>for</strong> reaction 2<br />
Evaluation: reaction 1, ABB; reaction 2, AAA<br />
Structure:<br />
Values from literature<br />
pK rH°/(kJ mol 1 ) T/K I Methods and comments Reference<br />
Reaction „1…: H 2LÄH ¿ ¿HL À<br />
1.40 293.15 0.05 M Electrometric titration—hydrogen electrode. 26MEY/SUR<br />
1.36 291.15 0.06 M Potentiometric titration—glass electrode. 30MOR<br />
1.37 298.15 0.06 M Electrometric titration—hydrogen electrode. The temperature was<br />
ambient and is assumed to be 298.15 K.<br />
34KIE<br />
1.221<br />
1.252<br />
278.15<br />
283.15<br />
0<br />
0<br />
Electrochemical cell—no liquid junction. The value <strong>of</strong> rH° given<br />
here was calculated from pKs measured at several temperatures. The<br />
54ASH/CRO<br />
1.272<br />
1.296<br />
288.15<br />
293.15<br />
0<br />
0<br />
1 1 value rC p/(J K mol )330 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong> reported 54ASH/CRO pKs.<br />
1.329 12.18 298.15 0<br />
1.373 303.15 0<br />
1.416 308.15 0<br />
1.460 313.15 0<br />
1.509 318.15 0<br />
1.549 323.15 0<br />
Reaction „2…: HLÀÄH ¿ ¿L2À 6.33 293.15 0.07 M Electrometric titration—hydrogen electrode. 26MEY/SUR<br />
6.67 0 303.15 0 Electrochemical cell with liquid junction—hydrogen and calomel<br />
electrodes. The results have been extrapolated to I0.<br />
28MOR<br />
6.32 291.15 0.09 M Potentiometric titration—glass electrode. 30MOR<br />
6.34 298.15 0.09 M Electrometric titration—hydrogen electrode. The temperature was<br />
ambient and is assumed to be 298.15 K.<br />
34KIE<br />
6.34 302.65 0.02 M Potentiometric titration—hydrogen and calomel electrodes. 39KIN/DEL<br />
6.657<br />
6.650<br />
278.15<br />
283.15<br />
0<br />
0<br />
Titrations were per<strong>for</strong>med at T295.15 K and at T310.15 K.<br />
Since only a mean value <strong>of</strong> <strong>the</strong> pK is reported, we have assigned a<br />
mean temperature <strong>of</strong> 302.65 K. The same result was again reported<br />
by Delory and King 43DEL/KIN.<br />
Electrochemical cell—no liquid junction. The value <strong>of</strong> rH° given<br />
here was calculated from pKs measured at several temperatures. The<br />
54ASH/CRO<br />
6.646<br />
6.647<br />
288.15<br />
293.15<br />
0<br />
0<br />
1 1 value rC p/(J K mol )228 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong> reported 54ASH/CRO pKs.<br />
6.650 1.74 298.15 0<br />
6.655 303.15 0<br />
6.667 308.15 0<br />
6.678 313.15 0<br />
J. Phys. Chem. Ref. Data, Vol. 31, No. 2, 2002