Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
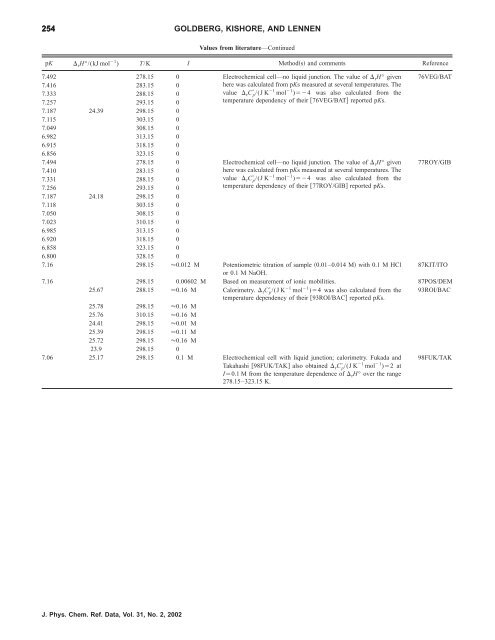
254 GOLDBERG, KISHORE, AND LENNEN<br />
Values from literature—Continued<br />
pK rH°/(kJ mol 1 ) T/K I Methods and comments Reference<br />
7.492 278.15 0 Electrochemical cell—no liquid junction. The value <strong>of</strong> rH° given<br />
7.416 283.15 0<br />
7.333 288.15 0<br />
7.257 293.15 0<br />
7.187 24.39 298.15 0<br />
7.115 303.15 0<br />
7.049 308.15 0<br />
6.982 313.15 0<br />
6.915 318.15 0<br />
6.856 323.15 0<br />
here was calculated from pKs measured at several temperatures. The<br />
value rC p /(J K 1 mol 1 )4 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 76VEG/BAT reported pKs.<br />
7.494 278.15 0 Electrochemical cell—no liquid junction. The value <strong>of</strong> rH° given<br />
7.410 283.15 0<br />
7.331 288.15 0<br />
7.256 293.15 0<br />
7.187 24.18 298.15 0<br />
7.118 303.15 0<br />
7.050 308.15 0<br />
7.023 310.15 0<br />
6.985 313.15 0<br />
6.920 318.15 0<br />
6.858 323.15 0<br />
6.800 328.15 0<br />
here was calculated from pKs measured at several temperatures. The<br />
value rC p /(J K 1 mol 1 )4 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 77ROY/GIB reported pKs.<br />
76VEG/BAT<br />
77ROY/GIB<br />
7.16 298.15 0.012 M Potentiometric titration <strong>of</strong> sample 0.01–0.014 M with 0.1 M HCl<br />
or 0.1 M NaOH.<br />
87KIT/ITO<br />
7.16 298.15 0.00602 M Based on measurement <strong>of</strong> ionic mobilities. 87POS/DEM<br />
25.67 288.15 0.16 M<br />
1 1 Calorimetry. rC p/(J K mol )4 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 93ROI/BAC reported pKs.<br />
93ROI/BAC<br />
25.78 298.15 0.16 M<br />
25.76 310.15 0.16 M<br />
24.41 298.15 0.01 M<br />
25.39 298.15 0.11 M<br />
25.72 298.15 0.16 M<br />
23.9 298.15 0<br />
7.06 25.17 298.15 0.1 M Electrochemical cell with liquid junction; calorimetry. Fukada and<br />
Takahashi 98FUK/TAK also obtained rC p /(J K 1 mol 1 )2 at<br />
I0.1 M from <strong>the</strong> temperature dependence <strong>of</strong> rH° over <strong>the</strong> range<br />
278.15–323.15 K.<br />
J. Phys. Chem. Ref. Data, Vol. 31, No. 2, 2002<br />
98FUK/TAK