Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
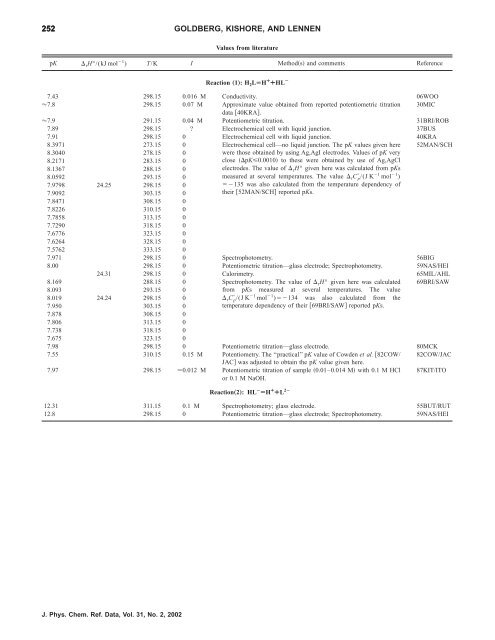
252 GOLDBERG, KISHORE, AND LENNEN<br />
Values from literature<br />
pK rH°/(kJ mol 1 ) T/K I Methods and comments Reference<br />
Reaction „1…: H 2LÄH ¿ ¿HL À<br />
7.43 298.15 0.016 M Conductivity. 06WOO<br />
7.8 298.15 0.07 M Approximate value obtained from reported potentiometric titration<br />
data 40KRA.<br />
30MIC<br />
7.9 291.15 0.04 M Potentiometric titration. 31BRI/ROB<br />
7.89 298.15 ? Electrochemical cell with liquid junction. 37BUS<br />
7.91 298.15 0 Electrochemical cell with liquid junction. 40KRA<br />
8.3971 273.15 0 Electrochemical cell—no liquid junction. The pK values given here 52MAN/SCH<br />
8.3040 278.15 0<br />
were those obtained by using Ag,AgI electrodes. Values <strong>of</strong> pK very<br />
8.2171 283.15 0<br />
close pK0.0010 to <strong>the</strong>se were obtained by use <strong>of</strong> Ag,AgCl<br />
8.1367<br />
8.0592<br />
7.9798 24.25<br />
288.15<br />
293.15<br />
298.15<br />
0<br />
0<br />
0<br />
electrodes. The value <strong>of</strong> rH° given here was calculated from pKs<br />
1 1 measured at several temperatures. The value rC p/(J K mol )<br />
135 was also calculated from <strong>the</strong> temperature dependency <strong>of</strong><br />
7.9092 303.15 0<br />
<strong>the</strong>ir 52MAN/SCH reported pKs.<br />
7.8471 308.15 0<br />
7.8226 310.15 0<br />
7.7858 313.15 0<br />
7.7290 318.15 0<br />
7.6776 323.15 0<br />
7.6264 328.15 0<br />
7.5762 333.15 0<br />
7.971 298.15 0 Spectrophotometry. 56BIG<br />
8.00 298.15 0 Potentiometric titration—glass electrode; Spectrophotometry. 59NAS/HEI<br />
24.31 298.15 0 Calorimetry. 65MIL/AHL<br />
8.169<br />
8.093<br />
288.15<br />
293.15<br />
0<br />
0<br />
Spectrophotometry. The value <strong>of</strong> rH° given here was calculated<br />
from pKs measured at several temperatures. The value<br />
69BRI/SAW<br />
8.019<br />
7.950<br />
24.24 298.15<br />
303.15<br />
0<br />
0<br />
1 1 rC p/(J K mol )134 was also calculated from<br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 69BRI/SAW reported pKs.<br />
<strong>the</strong><br />
7.878 308.15 0<br />
7.806 313.15 0<br />
7.738 318.15 0<br />
7.675 323.15 0<br />
7.98 298.15 0 Potentiometric titration—glass electrode. 80MCK<br />
7.55 310.15 0.15 M Potentiometry. The ‘‘practical’’ pK value <strong>of</strong> Cowden et al. 82COW/<br />
JAC was adjusted to obtain <strong>the</strong> pK value given here.<br />
82COW/JAC<br />
7.97 298.15 0.012 M Potentiometric titration <strong>of</strong> sample 0.01–0.014 M with 0.1 M HCl<br />
or 0.1 M NaOH.<br />
87KIT/ITO<br />
Reaction„2…: HL À ÄH ¿ ¿L 2À<br />
12.31 311.15 0.1 M Spectrophotometry; glass electrode. 55BUT/RUT<br />
12.8 298.15 0 Potentiometric titration—glass electrode; Spectrophotometry. 59NAS/HEI<br />
J. Phys. Chem. Ref. Data, Vol. 31, No. 2, 2002