Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
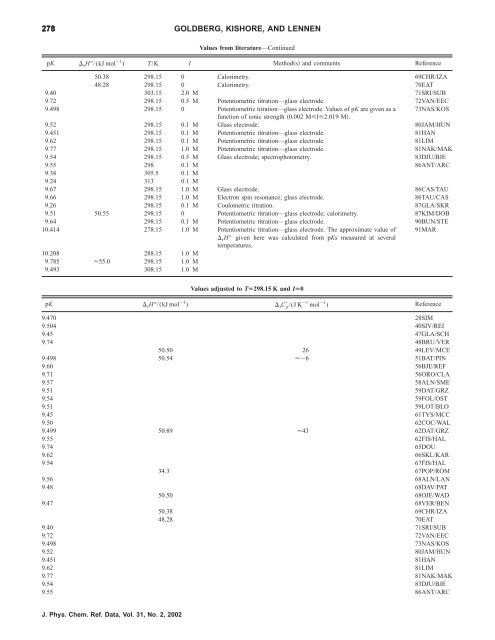
278 GOLDBERG, KISHORE, AND LENNEN<br />
Values from literature—Continued<br />
pK rH°/(kJ mol 1 ) T/K I Methods and comments Reference<br />
50.38 298.15 0 Calorimetry. 69CHR/IZA<br />
48.28 298.15 0 Calorimetry. 70EAT<br />
9.40 303.15 2.0 M 71SRI/SUB<br />
9.72 298.15 0.5 M Potentiometric titration—glass electrode. 72VAN/EEC<br />
9.498 298.15 0 Potentiometric titration—glass electrode. Values <strong>of</strong> pK are given as a<br />
function <strong>of</strong> ionic strength 0.002 MI2.019 M.<br />
73NAS/KOS<br />
9.52 298.15 0.1 M Glass electrode. 80JAM/HUN<br />
9.451 298.15 0.1 M Potentiometric titration—glass electrode. 81HAN<br />
9.62 298.15 0.1 M Potentiometric titration—glass electrode. 81LIM<br />
9.77 298.15 1.0 M Potentiometric titration—glass electrode. 81NAK/MAK<br />
9.54 298.15 0.5 M Glass electrode; spectrophotometry. 83DJU/BJE<br />
9.55 298 0.1 M 86ANT/ARC<br />
9.38 305.5 0.1 M<br />
9.24 313 0.1 M<br />
9.67 298.15 1.0 M Glass electrode. 86CAS/TAU<br />
9.66 298.15 1.0 M Electron spin resonance; glass electrode. 86TAU/CAS<br />
9.26 298.15 0.1 M Coulometric titration. 87GLA/SKR<br />
9.51 50.55 298.15 0 Potentiometric titration—glass electrode; calorimetry. 87KIM/DOB<br />
9.64 298.15 0.1 M Potentiometric titration—glass electrode. 90BUN/STE<br />
10.414 278.15 1.0 M Potentiometric titration—glass electrode. The approximate value <strong>of</strong><br />
rH° given here was calculated from pKs measured at several<br />
temperatures.<br />
91MAR<br />
10.208 288.15 1.0 M<br />
9.785 55.0 298.15 1.0 M<br />
9.493 308.15 1.0 M<br />
Values adjusted to TÄ298.15 K and IÄ0<br />
pK rH°/(kJ mol 1 ) rC p /(J K 1 mol 1 ) Reference<br />
9.470 28SIM<br />
9.504 40SIV/REI<br />
9.45 47GLA/SCH<br />
9.74 48BRU/VER<br />
50.50 26 49LEV/MCE<br />
9.498 50.54 6 51BAT/PIN<br />
9.60 56BJE/REF<br />
9.71 56ORO/CLA<br />
9.57 58ALN/SME<br />
9.51 59DAT/GRZ<br />
9.54 59FOL/OST<br />
9.51 59LOT/BLO<br />
9.45 61TYS/MCC<br />
9.50 62COC/WAL<br />
9.499 50.89 43 62DAT/GRZ<br />
9.55 62FIS/HAL<br />
9.74 65DOU<br />
9.62 66SKL/KAR<br />
9.54 67FIS/HAL<br />
34.3 67POP/ROM<br />
9.56 68ALN/LAN<br />
9.48 68DAV/PAT<br />
50.50 68OJE/WAD<br />
9.47 68VER/BEN<br />
50.38 69CHR/IZA<br />
48.28 70EAT<br />
9.40 71SRI/SUB<br />
9.72 72VAN/EEC<br />
9.498 73NAS/KOS<br />
9.52 80JAM/HUN<br />
9.451 81HAN<br />
9.62 81LIM<br />
9.77 81NAK/MAK<br />
9.54 83DJU/BJE<br />
9.55 86ANT/ARC<br />
J. Phys. Chem. Ref. Data, Vol. 31, No. 2, 2002