Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
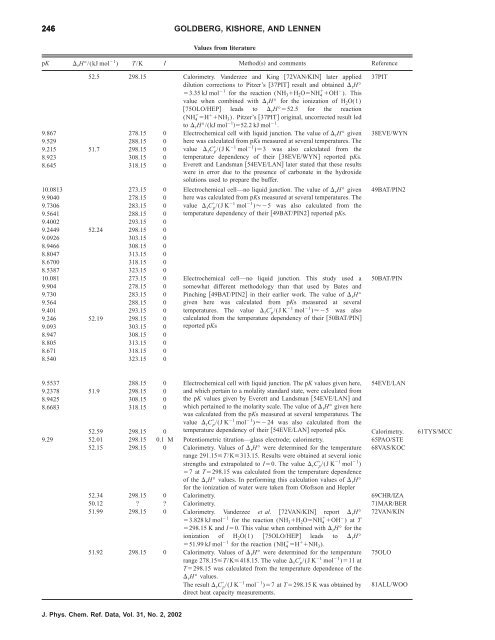
246 GOLDBERG, KISHORE, AND LENNEN<br />
Values from literature<br />
pK rH°/(kJ mol 1 ) T/K I Methods and comments Reference<br />
52.5 298.15 Calorimetry. Vanderzee and King 72VAN/KIN later applied<br />
dilution corrections to Pitzer’s 37PIT result and obtained rH°<br />
3.35 kJ mol 1 <strong>for</strong> <strong>the</strong> reaction (NH 3H 2ONH 4 OH ). This<br />
value when combined with rH° <strong>for</strong> <strong>the</strong> ionization <strong>of</strong> H 2O(1)<br />
75OLO/HEP leads to rH°52.5 <strong>for</strong> <strong>the</strong> reaction<br />
(NH 4 H NH3). Pitzer’s 37PIT original, uncorrected result led<br />
to rH°/(kJ mol 1 52.2 kJ mol 1 .<br />
9.867 278.15 0 Electrochemical cell with liquid junction. The value <strong>of</strong> rH° given<br />
9.529 288.15 0<br />
9.215 51.7 298.15 0<br />
8.923 308.15 0<br />
8.645 318.15 0<br />
here was calculated from pKs measured at several temperatures. The<br />
value rC p /(J K 1 mol 1 )3 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 38EVE/WYN reported pKs.<br />
Everett and Landsman 54EVE/LAN later stated that <strong>the</strong>se results<br />
were in error due to <strong>the</strong> presence <strong>of</strong> carbonate in <strong>the</strong> hydroxide<br />
solutions used to prepare <strong>the</strong> buffer.<br />
10.0813 273.15 0 Electrochemical cell—no liquid junction. The value <strong>of</strong> rH° given<br />
9.9040 278.15 0<br />
9.7306 283.15 0<br />
9.5641 288.15 0<br />
9.4002 293.15 0<br />
9.2449 52.24 298.15 0<br />
9.0926 303.15 0<br />
8.9466 308.15 0<br />
8.8047 313.15 0<br />
8.6700 318.15 0<br />
8.5387 323.15 0<br />
here was calculated from pKs measured at several temperatures. The<br />
value rC p /(J K 1 mol 1 )5 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 49BAT/PIN2 reported pKs.<br />
10.081 273.15 0 Electrochemical cell—no liquid junction. This study used a<br />
9.904 278.15 0<br />
9.730 283.15 0<br />
9.564 288.15 0<br />
9.401 293.15 0<br />
9.246 52.19 298.15 0<br />
9.093 303.15 0<br />
8.947 308.15 0<br />
8.805 313.15 0<br />
8.671 318.15 0<br />
8.540 323.15 0<br />
somewhat different methodology than that used by Bates and<br />
Pinching 49BAT/PIN2 in <strong>the</strong>ir earlier work. The value <strong>of</strong> rH°<br />
given here was calculated from pKs measured at several<br />
temperatures. The value rC p /(J K 1 mol 1 )5 was also<br />
calculated from <strong>the</strong> temperature dependency <strong>of</strong> <strong>the</strong>ir 50BAT/PIN<br />
reported pKs<br />
9.5537 288.15 0 Electrochemical cell with liquid junction. The pK values given here,<br />
9.2378 51.9 298.15 0<br />
8.9425 308.15 0<br />
8.6683 318.15 0<br />
and which pertain to a molality standard state, were calculated from<br />
<strong>the</strong> pK values given by Everett and Landsman 54EVE/LAN and<br />
which pertained to <strong>the</strong> molarity scale. The value <strong>of</strong> rH° given here<br />
was calculated from <strong>the</strong> pKs measured at several temperatures. The<br />
value rC p /(J K 1 mol 1 )24 was also calculated from <strong>the</strong><br />
37PIT<br />
38EVE/WYN<br />
49BAT/PIN2<br />
50BAT/PIN<br />
54EVE/LAN<br />
52.59 298.15 0 temperature dependency <strong>of</strong> <strong>the</strong>ir 54EVE/LAN reported pKs. Calorimetry. 61TYS/MCC<br />
9.29 52.01 298.15 0.1 M Potentiometric titration—glass electrode; calorimetry. 65PAO/STE<br />
68VAS/KOC<br />
52.15 298.15 0 Calorimetry. Values <strong>of</strong> rH° were determined <strong>for</strong> <strong>the</strong> temperature<br />
range 291.15T/K313.15. Results were obtained at several ionic<br />
strengths and extrapolated to I0. The value rC p /(J K 1 mol 1 )<br />
7 atT298.15 was calculated from <strong>the</strong> temperature dependence<br />
<strong>of</strong> <strong>the</strong> rH° values. In per<strong>for</strong>ming this calculation values <strong>of</strong> rH°<br />
52.34 298.15 0<br />
<strong>for</strong> <strong>the</strong> ionization <strong>of</strong> water were taken from Ol<strong>of</strong>sson and Hepler<br />
Calorimetry. 69CHR/IZA<br />
50.12 ? ? Calorimetry. 71MAR/BER<br />
51.99 298.15 0 Calorimetry. Vanderzee et al. 72VAN/KIN report rH° 3.828 kJ mol1 <strong>for</strong> <strong>the</strong> reaction (NH3H2ONH4 OH ) at T<br />
298.15 K and I0. This value when combined with rH° <strong>for</strong> <strong>the</strong><br />
ionization <strong>of</strong> H2O(1) 75OLO/HEP leads<br />
51.99 kJ mol<br />
to rH° 1 72VAN/KIN<br />
<strong>for</strong> <strong>the</strong> reaction (NH4 H NH3).<br />
75OLO<br />
51.92 298.15 0 Calorimetry. Values <strong>of</strong> rH° were determined <strong>for</strong> <strong>the</strong> temperature<br />
range 278.15T/K418.15. The value rC p /(J K 1 mol 1 )11 at<br />
T298.15 was calculated from <strong>the</strong> temperature dependence <strong>of</strong> <strong>the</strong><br />
J. Phys. Chem. Ref. Data, Vol. 31, No. 2, 2002<br />
rH° values.<br />
The result rC p /(J K 1 mol 1 )7 atT298.15 K was obtained by<br />
direct heat capacity measurements.<br />
81ALL/WOO