Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
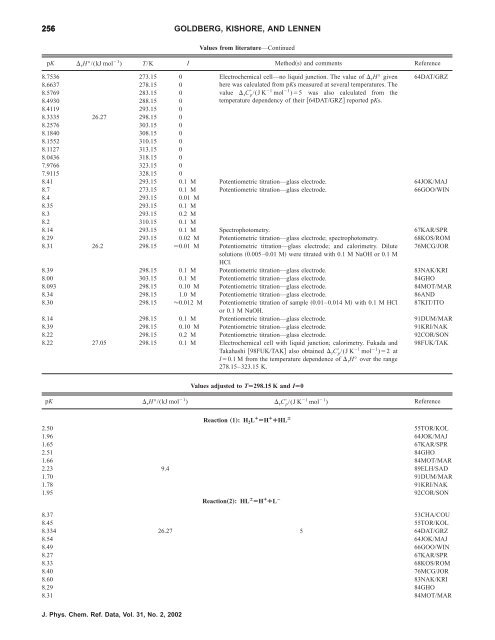
256 GOLDBERG, KISHORE, AND LENNEN<br />
Values from literature—Continued<br />
pK rH°/(kJ mol 1 ) T/K I Methods and comments Reference<br />
8.7536 273.15 0 Electrochemical cell—no liquid junction. The value <strong>of</strong> rH° given<br />
8.6637 278.15 0<br />
8.5769 283.15 0<br />
8.4930 288.15 0<br />
8.4119 293.15 0<br />
8.3335 26.27 298.15 0<br />
8.2576 303.15 0<br />
8.1840 308.15 0<br />
8.1552 310.15 0<br />
8.1127 313.15 0<br />
8.0436 318.15 0<br />
7.9766 323.15 0<br />
7.9115 328.15 0<br />
here was calculated from pKs measured at several temperatures. The<br />
value rC p /(J K 1 mol 1 )5 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 64DAT/GRZ reported pKs.<br />
64DAT/GRZ<br />
8.41 293.15 0.1 M Potentiometric titration—glass electrode. 64JOK/MAJ<br />
8.7 273.15 0.1 M Potentiometric titration—glass electrode. 66GOO/WIN<br />
8.4 293.15 0.01 M<br />
8.35 293.15 0.1 M<br />
8.3 293.15 0.2 M<br />
8.2 310.15 0.1 M<br />
8.14 293.15 0.1 M Spectrophotometry. 67KAR/SPR<br />
8.29 293.15 0.02 M Potentiometric titration—glass electrode; spectrophotometry. 68KOS/ROM<br />
8.31 26.2 298.15 0.01 M Potentiometric titration—glass electrode; and calorimetry. Dilute<br />
solutions 0.005–0.01 M were titrated with 0.1 M NaOH or 0.1 M<br />
HCl.<br />
76MCG/JOR<br />
8.39 298.15 0.1 M Potentiometric titration—glass electrode. 83NAK/KRI<br />
8.00 303.15 0.1 M Potentiometric titration—glass electrode. 84GHO<br />
8.093 298.15 0.10 M Potentiometric titration—glass electrode. 84MOT/MAR<br />
8.34 298.15 1.0 M Potentiometric titration—glass electrode. 86AND<br />
8.30 298.15 0.012 M Potentiometric titration <strong>of</strong> sample 0.01–0.014 M with 0.1 M HCl<br />
or 0.1 M NaOH.<br />
87KIT/ITO<br />
8.14 298.15 0.1 M Potentiometric titration—glass electrode. 91DUM/MAR<br />
8.39 298.15 0.10 M Potentiometric titration—glass electrode. 91KRI/NAK<br />
8.22 298.15 0.2 M Potentiometric titration—glass electrode. 92COR/SON<br />
8.22 27.05 298.15 0.1 M Electrochemical cell with liquid junction; calorimetry. Fukada and<br />
Takahashi 98FUK/TAK also obtained rC p /(J K 1 mol 1 )2 at<br />
I0.1 M from <strong>the</strong> temperature dependence <strong>of</strong> rH° over <strong>the</strong> range<br />
278.15–323.15 K.<br />
Values adjusted to TÄ298.15 K and IÄ0<br />
98FUK/TAK<br />
pK rH°/(kJ mol 1 ) rC p /(J K 1 mol 1 ) Reference<br />
Reaction „1…: H 2L ¿ ÄH ¿ ¿HL Á<br />
2.50 55TOR/KOL<br />
1.96 64JOK/MAJ<br />
1.65 67KAR/SPR<br />
2.51 84GHO<br />
1.66 84MOT/MAR<br />
2.23 9.4 89ELH/SAD<br />
1.70 91DUM/MAR<br />
1.78 91KRI/NAK<br />
1.95 92COR/SON<br />
Reaction„2…: HL Á ÄH ¿ ¿L À<br />
8.37 53CHA/COU<br />
8.45 55TOR/KOL<br />
8.334 26.27 5 64DAT/GRZ<br />
8.54 64JOK/MAJ<br />
8.49 66GOO/WIN<br />
8.27 67KAR/SPR<br />
8.33 68KOS/ROM<br />
8.40 76MCG/JOR<br />
8.60 83NAK/KRI<br />
8.29 84GHO<br />
8.31 84MOT/MAR<br />
J. Phys. Chem. Ref. Data, Vol. 31, No. 2, 2002