Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
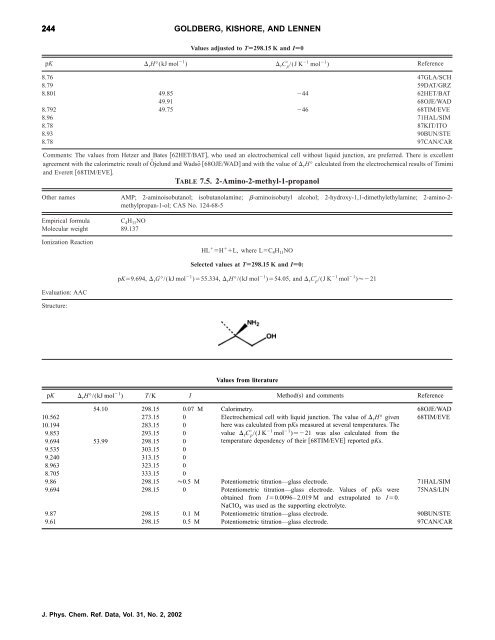
244 GOLDBERG, KISHORE, AND LENNEN<br />
Values adjusted to TÄ298.15 K and IÄ0<br />
pK rH°(kJ mol 1 ) rC p /(J K 1 mol 1 ) Reference<br />
8.76 47GLA/SCH<br />
8.79 59DAT/GRZ<br />
8.801 49.85 44 62HET/BAT<br />
49.91 68OJE/WAD<br />
8.792 49.75 46 68TIM/EVE<br />
8.96 71HAL/SIM<br />
8.78 87KIT/ITO<br />
8.93 90BUN/STE<br />
8.78 97CAN/CAR<br />
Comments: The values from Hetzer and Bates 62HET/BAT, who used an electrochemical cell without liquid junction, are preferred. There is excellent<br />
agreement with <strong>the</strong> calorimetric result <strong>of</strong> Öjelund and Wadsö 68OJE/WAD and with <strong>the</strong> value <strong>of</strong> rH° calculated from <strong>the</strong> electrochemical results <strong>of</strong> Timimi<br />
and Everett 68TIM/EVE.<br />
TABLE 7.5. 2-Amino-2-methyl-1-propanol<br />
O<strong>the</strong>r names AMP; 2-aminoisobutanol; isobutanolamine; -aminoisobutyl alcohol; 2-hydroxy-1,1-dimethylethylamine; 2-amino-2methylpropan-1-ol;<br />
CAS No. 124-68-5<br />
Empirical <strong>for</strong>mula C 4H 11NO<br />
Molecular weight 89.137<br />
<strong>Ionization</strong> Reaction<br />
Evaluation: AAC<br />
Structure:<br />
HL H L, where LC 4H 11NO<br />
Selected values at TÄ298.15 K and IÄ0:<br />
pK9.694, rG°/(kJ mol 1 )55.334, rH°/(kJ mol 1 )54.05, and rC p /(J K 1 mol 1 )21<br />
Values from literature<br />
pK rH°/(kJ mol 1 ) T/K I Methods and comments Reference<br />
54.10 298.15 0.07 M Calorimetry. 68OJE/WAD<br />
10.562<br />
10.194<br />
273.15<br />
283.15<br />
0<br />
0<br />
Electrochemical cell with liquid junction. The value <strong>of</strong> rH° given<br />
here was calculated from pKs measured at several temperatures. The<br />
68TIM/EVE<br />
9.853<br />
9.694 53.99<br />
293.15<br />
298.15<br />
0<br />
0<br />
1 1 value rC p/(J K mol )21 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 68TIM/EVE reported pKs.<br />
9.535 303.15 0<br />
9.240 313.15 0<br />
8.963 323.15 0<br />
8.705 333.15 0<br />
9.86 298.15 0.5 M Potentiometric titration—glass electrode. 71HAL/SIM<br />
9.694 298.15 0 Potentiometric titration—glass electrode. Values <strong>of</strong> pKs were 75NAS/LIN<br />
obtained from I0.0096– 2.019 M and extrapolated to I0.<br />
9.87 298.15 0.1 M<br />
NaClO4 was used as <strong>the</strong> supporting electrolyte.<br />
Potentiometric titration—glass electrode. 90BUN/STE<br />
9.61 298.15 0.5 M Potentiometric titration—glass electrode. 97CAN/CAR<br />
J. Phys. Chem. Ref. Data, Vol. 31, No. 2, 2002