Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
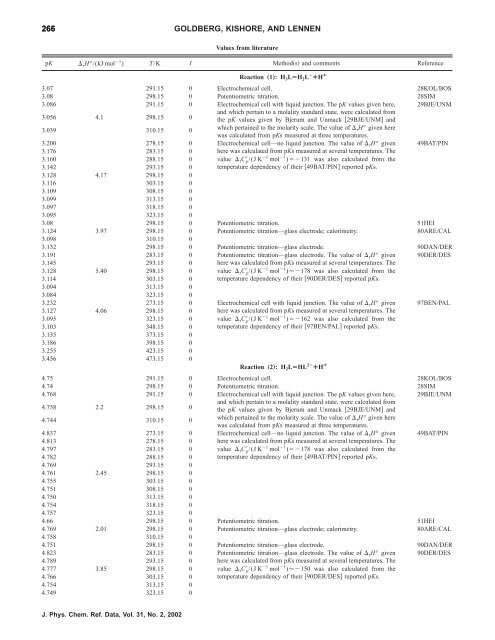
266 GOLDBERG, KISHORE, AND LENNEN<br />
Values from literature<br />
pK rH°/(kJ mol 1 ) T/K I Methods and comments Reference<br />
Reaction „1…: H3LÄH2LÀ ¿H ¿<br />
3.07 291.15 0 Electrochemical cell. 28KOL/BOS<br />
3.08 298.15 0 Potentiometric titration. 28SIM<br />
3.086 291.15 0 Electrochemical cell with liquid junction. The pK values given here, 29BJE/UNM<br />
3.056 4.1 298.15 0<br />
and which pertain to a molality standard state, were calculated from<br />
<strong>the</strong> pK values given by Bjerum and Unmack 29BJE/UNM and<br />
3.039 310.15 0<br />
which pertained to <strong>the</strong> molarity scale. The value <strong>of</strong> rH° given here<br />
was calculated from pKs measured at three temperatures.<br />
3.200<br />
3.176<br />
278.15<br />
283.15<br />
0<br />
0<br />
Electrochemical cell—no liquid junction. The value <strong>of</strong> rH° given<br />
here was calculated from pKs measured at several temperatures. The<br />
49BAT/PIN<br />
3.160<br />
3.142<br />
288.15<br />
293.15<br />
0<br />
0<br />
1 1 value rC p/(J K mol )131 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 49BAT/PIN reported pKs.<br />
3.128 4.17 298.15 0<br />
3.116 303.15 0<br />
3.109 308.15 0<br />
3.099 313.15 0<br />
3.097 318.15 0<br />
3.095 323.15 0<br />
3.08 298.15 0 Potentiometric titration. 51HEI<br />
3.124 3.97 298.15 0 Potentiometric titration—glass electrode; calorimetry. 80ARE/CAL<br />
3.098 310.15 0<br />
3.132 298.15 0 Potentiometric titration—glass electrode. 90DAN/DER<br />
3.191<br />
3.145<br />
283.15<br />
293.15<br />
0<br />
0<br />
Potentiometric titration—glass electrode. The value <strong>of</strong> rH° given<br />
here was calculated from pKs measured at several temperatures. The<br />
90DER/DES<br />
3.128<br />
3.114<br />
5.40 298.15<br />
303.15<br />
0<br />
0<br />
1 1 value rC p/(J K mol )178 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 90DER/DES reported pKs.<br />
3.094 313.15 0<br />
3.084 323.15 0<br />
3.232<br />
3.127 4.06<br />
273.15<br />
298.15<br />
0<br />
0<br />
Electrochemical cell with liquid junction. The value <strong>of</strong> rH° given<br />
here was calculated from pKs measured at several temperatures. The<br />
97BEN/PAL<br />
3.095<br />
3.103<br />
323.15<br />
348.15<br />
0<br />
0<br />
1 1 value rC p/(J K mol )162 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 97BEN/PAL reported pKs.<br />
3.135 373.15 0<br />
3.186 398.15 0<br />
3.255 423.15 0<br />
3.456 473.15 0<br />
Reaction „2…: H2LÄHL2À ¿H ¿<br />
4.75 291.15 0 Electrochemical cell. 28KOL/BOS<br />
4.74 298.15 0 Potentiometric titration. 28SIM<br />
4.768 291.15 0 Electrochemical cell with liquid junction. The pK values given here, 29BJE/UNM<br />
4.758 2.2 298.15 0<br />
and which pertain to a molality standard state, were calculated from<br />
<strong>the</strong> pK values given by Bjerum and Unmack 29BJE/UNM and<br />
4.744 310.15 0<br />
which pertained to <strong>the</strong> molarity scale. The value <strong>of</strong> rH° given here<br />
was calculated from pKs measured at three temperatures.<br />
4.837<br />
4.813<br />
273.15<br />
278.15<br />
0<br />
0<br />
Electrochemical cell—no liquid junction. The value <strong>of</strong> rH° given<br />
here was calculated from pKs measured at several temperatures. The<br />
49BAT/PIN<br />
4.797<br />
4.782<br />
283.15<br />
288.15<br />
0<br />
0<br />
1 1 value rC p/(J K mol )178 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 49BAT/PIN reported pKs.<br />
4.769 293.15 0<br />
4.761 2.45 298.15 0<br />
4.755 303.15 0<br />
4.751 308.15 0<br />
4.750 313.15 0<br />
4.754 318.15 0<br />
4.757 323.15 0<br />
4.66 298.15 0 Potentiometric titration. 51HEI<br />
4.769 2.01 298.15 0 Potentiometric titration—glass electrode; calorimetry. 80ARE/CAL<br />
4.758 310.15 0<br />
4.751 298.15 0 Potentiometric titration—glass electrode. 90DAN/DER<br />
4.823<br />
4.789<br />
283.15<br />
293.15<br />
0<br />
0<br />
Potentiometric titration—glass electrode. The value <strong>of</strong> rH° given<br />
here was calculated from pKs measured at several temperatures. The<br />
90DER/DES<br />
4.777<br />
4.766<br />
3.85 298.15<br />
303.15<br />
0<br />
0<br />
1 1 value rC p/(J K mol )150 was also calculated from <strong>the</strong><br />
temperature dependency <strong>of</strong> <strong>the</strong>ir 90DER/DES reported pKs.<br />
4.754 313.15 0<br />
4.749 323.15 0<br />
J. Phys. Chem. Ref. Data, Vol. 31, No. 2, 2002