Antiemetics for reducing vomiting related to acute ... - Update Software
Antiemetics for reducing vomiting related to acute ... - Update Software
Antiemetics for reducing vomiting related to acute ... - Update Software
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
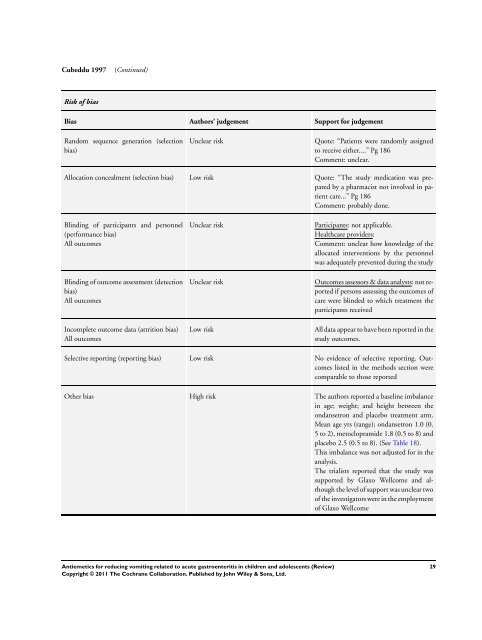
Cubeddu 1997 (Continued)<br />
Risk of bias<br />
Bias Authors’ judgement Support <strong>for</strong> judgement<br />
Random sequence generation (selection<br />
bias)<br />
Unclear risk Quote: “Patients were randomly assigned<br />
<strong>to</strong> receive either....” Pg 186<br />
Comment: unclear.<br />
Allocation concealment (selection bias) Low risk Quote: “The study medication was prepared<br />
by a pharmacist not involved in patient<br />
care...” Pg 186<br />
Comment: probably done.<br />
Blinding of participants and personnel<br />
(per<strong>for</strong>mance bias)<br />
All outcomes<br />
Blinding of outcome assessment (detection<br />
bias)<br />
All outcomes<br />
Incomplete outcome data (attrition bias)<br />
All outcomes<br />
Unclear risk Participants: not applicable.<br />
Healthcare providers:<br />
Comment: unclear how knowledge of the<br />
allocated interventions by the personnel<br />
was adequately prevented during the study<br />
Unclear risk Outcomes assessors & data analysts: not reported<br />
if persons assessing the outcomes of<br />
care were blinded <strong>to</strong> which treatment the<br />
participants received<br />
Low risk All data appear <strong>to</strong> have been reported in the<br />
study outcomes.<br />
Selective reporting (reporting bias) Low risk No evidence of selective reporting. Outcomes<br />
listed in the methods section were<br />
comparable <strong>to</strong> those reported<br />
Other bias High risk The authors reported a baseline imbalance<br />
in age; weight; and height between the<br />
ondansetron and placebo treatment arm.<br />
Mean age yrs (range); ondansetron 1.0 (0.<br />
5 <strong>to</strong> 2), me<strong>to</strong>clopramide 1.8 (0.5 <strong>to</strong> 8) and<br />
placebo 2.5 (0.5 <strong>to</strong> 8). (See Table 18).<br />
This imbalance was not adjusted <strong>for</strong> in the<br />
analysis.<br />
The trialists reported that the study was<br />
supported by Glaxo Wellcome and although<br />
the level of support was unclear two<br />
of the investiga<strong>to</strong>rs were in the employment<br />
of Glaxo Wellcome<br />
<strong>Antiemetics</strong> <strong>for</strong> <strong>reducing</strong> <strong>vomiting</strong> <strong>related</strong> <strong>to</strong> <strong>acute</strong> gastroenteritis in children and adolescents (Review)<br />
Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.<br />
29