Antiemetics for reducing vomiting related to acute ... - Update Software
Antiemetics for reducing vomiting related to acute ... - Update Software
Antiemetics for reducing vomiting related to acute ... - Update Software
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
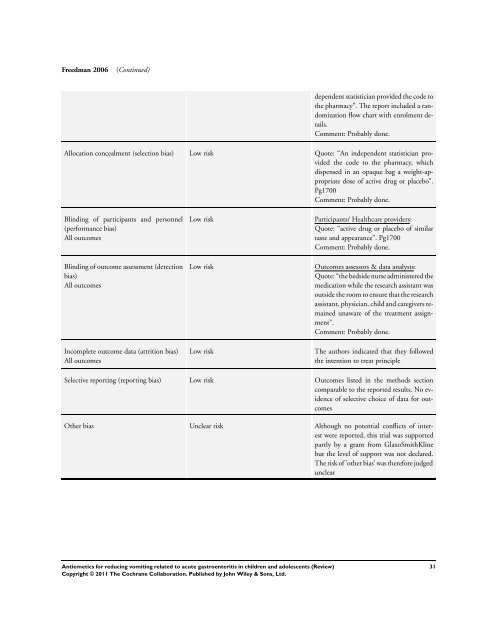
Freedman 2006 (Continued)<br />
dependent statistician provided the code <strong>to</strong><br />
the pharmacy”. The report included a randomization<br />
flow chart with enrolment details.<br />
Comment: Probably done.<br />
Allocation concealment (selection bias) Low risk Quote: “An independent statistician provided<br />
the code <strong>to</strong> the pharmacy, which<br />
dispensed in an opaque bag a weight-appropriate<br />
dose of active drug or placebo”.<br />
Pg1700<br />
Comment: Probably done.<br />
Blinding of participants and personnel<br />
(per<strong>for</strong>mance bias)<br />
All outcomes<br />
Blinding of outcome assessment (detection<br />
bias)<br />
All outcomes<br />
Incomplete outcome data (attrition bias)<br />
All outcomes<br />
Low risk Participants/ Healthcare providers:<br />
Quote: “active drug or placebo of similar<br />
taste and appearance”. Pg1700<br />
Comment: Probably done.<br />
Low risk Outcomes assessors & data analysts:<br />
Quote: “the bedside nurse administered the<br />
medication while the research assistant was<br />
outside the room <strong>to</strong> ensure that the research<br />
assistant, physician, child and caregivers remained<br />
unaware of the treatment assignment”.<br />
Comment: Probably done.<br />
Low risk The authors indicated that they followed<br />
the intention <strong>to</strong> treat principle<br />
Selective reporting (reporting bias) Low risk Outcomes listed in the methods section<br />
comparable <strong>to</strong> the reported results. No evidence<br />
of selective choice of data <strong>for</strong> outcomes<br />
Other bias Unclear risk Although no potential conflicts of interest<br />
were reported, this trial was supported<br />
partly by a grant from GlaxoSmithKline<br />
but the level of support was not declared.<br />
The risk of ’other bias’ was there<strong>for</strong>e judged<br />
unclear<br />
<strong>Antiemetics</strong> <strong>for</strong> <strong>reducing</strong> <strong>vomiting</strong> <strong>related</strong> <strong>to</strong> <strong>acute</strong> gastroenteritis in children and adolescents (Review)<br />
Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.<br />
31