Antiemetics for reducing vomiting related to acute ... - Update Software
Antiemetics for reducing vomiting related to acute ... - Update Software
Antiemetics for reducing vomiting related to acute ... - Update Software
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
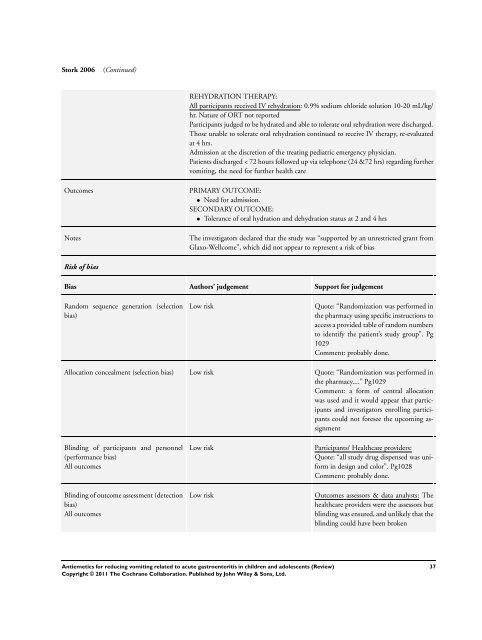
S<strong>to</strong>rk 2006 (Continued)<br />
REHYDRATION THERAPY:<br />
All participants received IV rehydration: 0.9% sodium chloride solution 10-20 mL/kg/<br />
hr. Nature of ORT not reported<br />
Participants judged <strong>to</strong> be hydrated and able <strong>to</strong> <strong>to</strong>lerate oral rehydration were discharged.<br />
Those unable <strong>to</strong> <strong>to</strong>lerate oral rehydration continued <strong>to</strong> receive IV therapy, re-evaluated<br />
at 4 hrs.<br />
Admission at the discretion of the treating pediatric emergency physician.<br />
Patients discharged < 72 hours followed up via telephone (24 &72 hrs) regarding further<br />
<strong>vomiting</strong>, the need <strong>for</strong> further health care<br />
Outcomes PRIMARY OUTCOME:<br />
• Need <strong>for</strong> admission.<br />
SECONDARY OUTCOME:<br />
• Tolerance of oral hydration and dehydration status at 2 and 4 hrs<br />
Notes The investiga<strong>to</strong>rs declared that the study was “supported by an unrestricted grant from<br />
Glaxo-Wellcome”, which did not appear <strong>to</strong> represent a risk of bias<br />
Risk of bias<br />
Bias Authors’ judgement Support <strong>for</strong> judgement<br />
Random sequence generation (selection<br />
bias)<br />
Low risk Quote: “Randomization was per<strong>for</strong>med in<br />
the pharmacy using specific instructions <strong>to</strong><br />
access a provided table of random numbers<br />
<strong>to</strong> identify the patient’s study group”. Pg<br />
1029<br />
Comment: probably done.<br />
Allocation concealment (selection bias) Low risk Quote: “Randomization was per<strong>for</strong>med in<br />
the pharmacy....” Pg1029<br />
Comment: a <strong>for</strong>m of central allocation<br />
was used and it would appear that participants<br />
and investiga<strong>to</strong>rs enrolling participants<br />
could not <strong>for</strong>esee the upcoming assignment<br />
Blinding of participants and personnel<br />
(per<strong>for</strong>mance bias)<br />
All outcomes<br />
Blinding of outcome assessment (detection<br />
bias)<br />
All outcomes<br />
Low risk Participants/ Healthcare providers:<br />
Quote: “all study drug dispensed was uni<strong>for</strong>m<br />
in design and color”. Pg1028<br />
Comment: probably done.<br />
Low risk Outcomes assessors & data analysts: The<br />
healthcare providers were the assessors but<br />
blinding was ensured, and unlikely that the<br />
blinding could have been broken<br />
<strong>Antiemetics</strong> <strong>for</strong> <strong>reducing</strong> <strong>vomiting</strong> <strong>related</strong> <strong>to</strong> <strong>acute</strong> gastroenteritis in children and adolescents (Review)<br />
Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.<br />
37