Antiemetics for reducing vomiting related to acute ... - Update Software
Antiemetics for reducing vomiting related to acute ... - Update Software
Antiemetics for reducing vomiting related to acute ... - Update Software
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
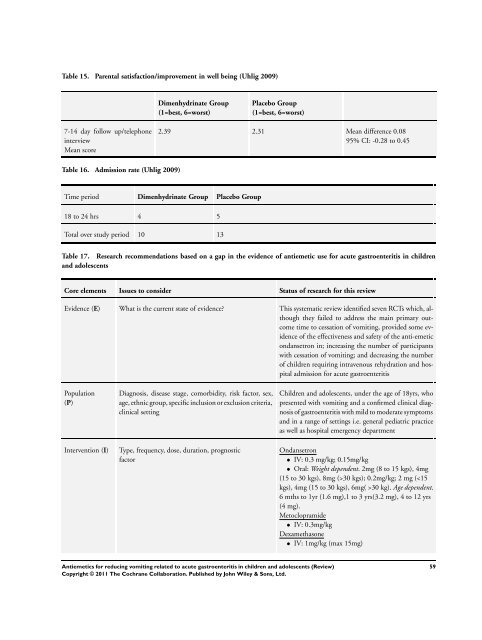
Table 15. Parental satisfaction/improvement in well being (Uhlig 2009)<br />
7-14 day follow up/telephone<br />
interview<br />
Mean score<br />
Table 16. Admission rate (Uhlig 2009)<br />
Dimenhydrinate Group<br />
(1=best, 6=worst)<br />
Placebo Group<br />
(1=best, 6=worst)<br />
2.39 2.31 Mean difference 0.08<br />
95% CI: -0.28 <strong>to</strong> 0.45<br />
Time period Dimenhydrinate Group Placebo Group<br />
18 <strong>to</strong> 24 hrs 4 5<br />
Total over study period 10 13<br />
Table 17. Research recommendations based on a gap in the evidence of antiemetic use <strong>for</strong> <strong>acute</strong> gastroenteritis in children<br />
and adolescents<br />
Core elements Issues <strong>to</strong> consider Status of research <strong>for</strong> this review<br />
Evidence (E) What is the current state of evidence? This systematic review identified seven RCTs which, although<br />
they failed <strong>to</strong> address the main primary outcome<br />
time <strong>to</strong> cessation of <strong>vomiting</strong>, provided some evidence<br />
of the effectiveness and safety of the anti-emetic<br />
ondansetron in; increasing the number of participants<br />
with cessation of <strong>vomiting</strong>; and decreasing the number<br />
of children requiring intravenous rehydration and hospital<br />
admission <strong>for</strong> <strong>acute</strong> gastroenteritis<br />
Population<br />
(P)<br />
Diagnosis, disease stage, comorbidity, risk fac<strong>to</strong>r, sex,<br />
age, ethnic group, specific inclusion or exclusion criteria,<br />
clinical setting<br />
Intervention (I) Type, frequency, dose, duration, prognostic<br />
fac<strong>to</strong>r<br />
<strong>Antiemetics</strong> <strong>for</strong> <strong>reducing</strong> <strong>vomiting</strong> <strong>related</strong> <strong>to</strong> <strong>acute</strong> gastroenteritis in children and adolescents (Review)<br />
Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.<br />
Children and adolescents, under the age of 18yrs, who<br />
presented with <strong>vomiting</strong> and a confirmed clinical diagnosis<br />
of gastroenteritis with mild <strong>to</strong> moderate symp<strong>to</strong>ms<br />
and in a range of settings i.e. general pediatric practice<br />
as well as hospital emergency department<br />
Ondansetron<br />
• IV: 0.3 mg/kg; 0.15mg/kg<br />
• Oral: Weight dependent. 2mg (8 <strong>to</strong> 15 kgs), 4mg<br />
(15 <strong>to</strong> 30 kgs), 8mg (>30 kgs); 0.2mg/kg; 2 mg (30 kg). Age dependent.<br />
6 mths <strong>to</strong> 1yr (1.6 mg),1 <strong>to</strong> 3 yrs(3.2 mg), 4 <strong>to</strong> 12 yrs<br />
(4 mg).<br />
Me<strong>to</strong>clopramide<br />
• IV: 0.3mg/kg<br />
Dexamethasone<br />
• IV: 1mg/kg (max 15mg)<br />
59