The report is available in English with a French summary - KCE
The report is available in English with a French summary - KCE
The report is available in English with a French summary - KCE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
42 Plasma <strong>KCE</strong> Reports 120<br />
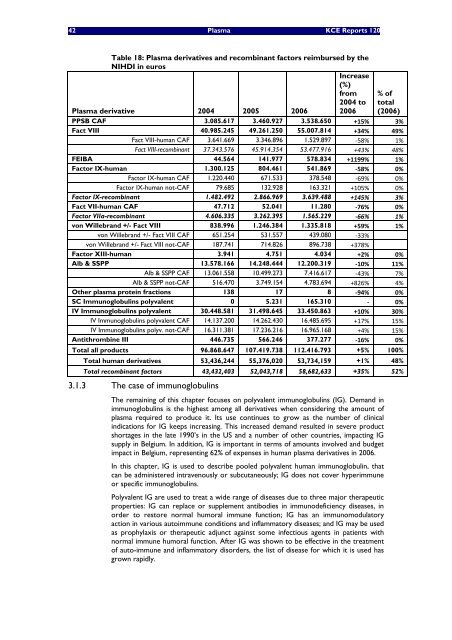
Table 18: Plasma derivatives and recomb<strong>in</strong>ant factors reimbursed by the<br />
NIHDI <strong>in</strong> euros<br />
Increase<br />
(%)<br />
from % of<br />
2004 to total<br />
Plasma derivative 2004 2005 2006 2006 (2006)<br />
PPSB CAF 3.085.617 3.460.927 3.538.650 +15% 3%<br />
Fact VIII 40.985.245 49.261.250 55.007.814 +34% 49%<br />
Fact VIII-human CAF 3.641.669 3.346.896 1.529.897 ‐58% 1%<br />
Fact VIII-recomb<strong>in</strong>ant 37.343.576 45.914.354 53.477.916 +43% 48%<br />
FEIBA 44.564 141.977 578.834 +1199% 1%<br />
Factor IX-human 1.300.125 804.461 541.869 ‐58% 0%<br />
Factor IX-human CAF 1.220.440 671.533 378.548 ‐69% 0%<br />
Factor IX-human not-CAF 79.685 132.928 163.321 +105% 0%<br />
Factor IX-recomb<strong>in</strong>ant 1.482.492 2.866.969 3.639.488 +145% 3%<br />
Fact VII-human CAF 47.712 52.041 11.280 ‐76% 0%<br />
Factor VIIa-recomb<strong>in</strong>ant 4.606.335 3.262.395 1.565.229 ‐66% 1%<br />
von Willebrand +/- Fact VIII 838.996 1.246.384 1.335.818 +59% 1%<br />
von Willebrand +/- Fact VIII CAF 651.254 531.557 439.080 ‐33%<br />
von Willebrand +/- Fact VIII not-CAF 187.741 714.826 896.738 +378%<br />
Factor XIII-human 3.941 4.751 4.034 +2% 0%<br />
Alb & SSPP 13.578.166 14.248.444 12.200.319 ‐10% 11%<br />
Alb & SSPP CAF 13.061.558 10.499.273 7.416.617 ‐43% 7%<br />
Alb & SSPP not-CAF 516.470 3.749.154 4.783.694 +826% 4%<br />
Other plasma prote<strong>in</strong> fractions 138 17 8 ‐94% 0%<br />
SC Immunoglobul<strong>in</strong>s polyvalent 0 5.231 165.310 ‐ 0%<br />
IV Immunoglobul<strong>in</strong>s polyvalent 30.448.581 31.498.645 33.450.863 +10% 30%<br />
IV Immunoglobul<strong>in</strong>s polyvalent CAF 14.137.200 14.262.430 16.485.695 +17% 15%<br />
IV Immunoglobul<strong>in</strong>s polyv. not-CAF 16.311.381 17.236.216 16.965.168 +4% 15%<br />
Antithromb<strong>in</strong>e III 446.735 566.246 377.277 ‐16% 0%<br />
Total all products 96.868.647 107.419.738 112.416.793 +5% 100%<br />
Total human derivatives 53,436,244 55,376,020 53,734,159 +1% 48%<br />
Total recomb<strong>in</strong>ant factors 43,432,403 52,043,718 58,682,633 +35% 52%<br />
3.1.3 <strong>The</strong> case of immunoglobul<strong>in</strong>s<br />
<strong>The</strong> rema<strong>in</strong><strong>in</strong>g of th<strong>is</strong> chapter focuses on polyvalent immunoglobul<strong>in</strong>s (IG). Demand <strong>in</strong><br />
immunoglobul<strong>in</strong>s <strong>is</strong> the highest among all derivatives when consider<strong>in</strong>g the amount of<br />
plasma required to produce it. Its use cont<strong>in</strong>ues to grow as the number of cl<strong>in</strong>ical<br />
<strong>in</strong>dications for IG keeps <strong>in</strong>creas<strong>in</strong>g. Th<strong>is</strong> <strong>in</strong>creased demand resulted <strong>in</strong> severe product<br />
shortages <strong>in</strong> the late 1990’s <strong>in</strong> the US and a number of other countries, impact<strong>in</strong>g IG<br />
supply <strong>in</strong> Belgium. In addition, IG <strong>is</strong> important <strong>in</strong> terms of amounts <strong>in</strong>volved and budget<br />
impact <strong>in</strong> Belgium, represent<strong>in</strong>g 62% of expenses <strong>in</strong> human plasma derivatives <strong>in</strong> 2006.<br />
In th<strong>is</strong> chapter, IG <strong>is</strong> used to describe pooled polyvalent human immunoglobul<strong>in</strong>, that<br />
can be adm<strong>in</strong><strong>is</strong>tered <strong>in</strong>travenously or subcutaneously; IG does not cover hyperimmune<br />
or specific immunoglobul<strong>in</strong>s.<br />
Polyvalent IG are used to treat a wide range of d<strong>is</strong>eases due to three major therapeutic<br />
properties: IG can replace or supplement antibodies <strong>in</strong> immunodeficiency d<strong>is</strong>eases, <strong>in</strong><br />
order to restore normal humoral immune function; IG has an immunomodulatory<br />
action <strong>in</strong> various autoimmune conditions and <strong>in</strong>flammatory d<strong>is</strong>eases; and IG may be used<br />
as prophylax<strong>is</strong> or therapeutic adjunct aga<strong>in</strong>st some <strong>in</strong>fectious agents <strong>in</strong> patients <strong>with</strong><br />
normal immune humoral function. After IG was shown to be effective <strong>in</strong> the treatment<br />
of auto-immune and <strong>in</strong>flammatory d<strong>is</strong>orders, the l<strong>is</strong>t of d<strong>is</strong>ease for which it <strong>is</strong> used has<br />
grown rapidly.