The report is available in English with a French summary - KCE
The report is available in English with a French summary - KCE
The report is available in English with a French summary - KCE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
44 Plasma <strong>KCE</strong> Reports 120<br />
• Idiopathic thrombocytopenic purpura: <strong>in</strong> children and <strong>in</strong> adults present<strong>in</strong>g<br />
a high r<strong>is</strong>k of bleed<strong>in</strong>g or for those await<strong>in</strong>g surgery<br />
2. Neurological <strong>in</strong>dications<br />
• Guilla<strong>in</strong>-Barré syndrome, <strong>with</strong> specific criteria<br />
• Chronic <strong>in</strong>flammatory demyel<strong>in</strong>at<strong>in</strong>g polyradiculoneuropathy (CIDP), <strong>with</strong><br />
specific criteria<br />
• Multifocal motor neuropathy (MMN), <strong>with</strong> specific criteria<br />
3. Other <strong>in</strong>dications<br />
• Kawasaki d<strong>is</strong>ease<br />
• Treatment of the toxic shock syndrome (TSS) due to streptococcal<br />
<strong>in</strong>fections<br />
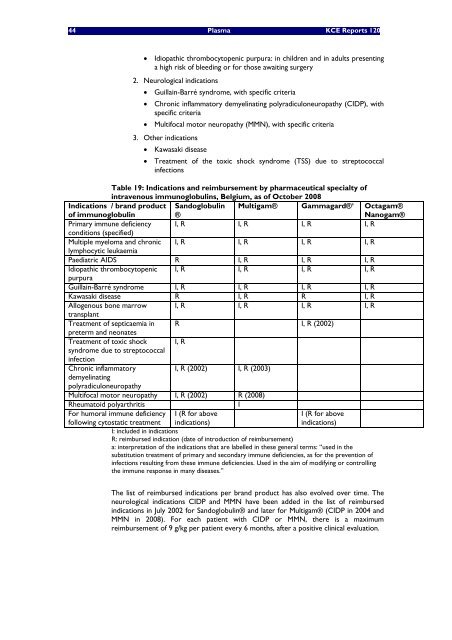
Table 19: Indications and reimbursement by pharmaceutical specialty of<br />
<strong>in</strong>travenous immunoglobul<strong>in</strong>s, Belgium, as of October 2008<br />
Indications / brand product Sandoglobul<strong>in</strong> Multigam® Gammagard®<br />
of immunoglobul<strong>in</strong><br />
®<br />
a Octagam®<br />
Nanogam®<br />
Primary immune deficiency<br />
conditions (specified)<br />
I, R I, R I, R I, R<br />
Multiple myeloma and chronic<br />
lymphocytic leukaemia<br />
I, R I, R I, R I, R<br />
Paediatric AIDS R I, R I, R I, R<br />
Idiopathic thrombocytopenic<br />
purpura<br />
I, R I, R I, R I, R<br />
Guilla<strong>in</strong>-Barré syndrome I, R I, R I, R I, R<br />
Kawasaki d<strong>is</strong>ease R I, R R I, R<br />
Allogenous bone marrow<br />
transplant<br />
I, R I, R I, R I, R<br />
Treatment of septicaemia <strong>in</strong><br />
preterm and neonates<br />
R I, R (2002)<br />
Treatment of toxic shock<br />
syndrome due to streptococcal<br />
<strong>in</strong>fection<br />
I, R<br />
Chronic <strong>in</strong>flammatory<br />
demyel<strong>in</strong>at<strong>in</strong>g<br />
polyradiculoneuropathy<br />
I, R (2002) I, R (2003)<br />
Multifocal motor neuropathy I, R (2002) R (2008)<br />
Rheumatoid polyarthrit<strong>is</strong> I<br />
For humoral immune deficiency I (R for above<br />
I (R for above<br />
follow<strong>in</strong>g cytostatic treatment <strong>in</strong>dications)<br />
<strong>in</strong>dications)<br />
I: <strong>in</strong>cluded <strong>in</strong> <strong>in</strong>dications<br />
R: reimbursed <strong>in</strong>dication (date of <strong>in</strong>troduction of reimbursement)<br />
a: <strong>in</strong>terpretation of the <strong>in</strong>dications that are labelled <strong>in</strong> these general terms: “used <strong>in</strong> the<br />
substitution treatment of primary and secondary immune deficiencies, as for the prevention of<br />
<strong>in</strong>fections result<strong>in</strong>g from these immune deficiencies. Used <strong>in</strong> the aim of modify<strong>in</strong>g or controll<strong>in</strong>g<br />
the immune response <strong>in</strong> many d<strong>is</strong>eases.”<br />
<strong>The</strong> l<strong>is</strong>t of reimbursed <strong>in</strong>dications per brand product has also evolved over time. <strong>The</strong><br />
neurological <strong>in</strong>dications CIDP and MMN have been added <strong>in</strong> the l<strong>is</strong>t of reimbursed<br />
<strong>in</strong>dications <strong>in</strong> July 2002 for Sandoglobul<strong>in</strong>® and later for Multigam® (CIDP <strong>in</strong> 2004 and<br />
MMN <strong>in</strong> 2008). For each patient <strong>with</strong> CIDP or MMN, there <strong>is</strong> a maximum<br />
reimbursement of 9 g/kg per patient every 6 months, after a positive cl<strong>in</strong>ical evaluation.