Cyanoguanidine CAS N°: 461-58-5
Cyanoguanidine CAS N°: 461-58-5
Cyanoguanidine CAS N°: 461-58-5
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
OECD SIDS<br />
CYANOGUANIDINE<br />
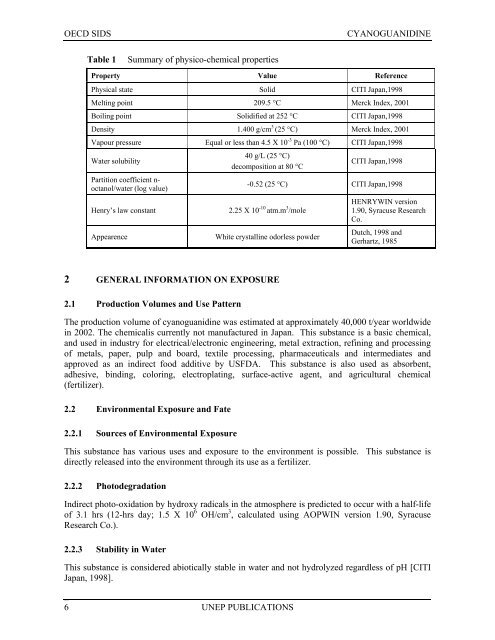
Table 1<br />
Summary of physico-chemical properties<br />
Property Value Reference<br />
Physical state Solid CITI Japan,1998<br />
Melting point 209.5 °C Merck Index, 2001<br />
Boiling point Solidified at 252 °C CITI Japan,1998<br />
Density 1.400 g/cm 3 (25 °C) Merck Index, 2001<br />
Vapour pressure Equal or less than 4.5 X 10 -3 Pa (100 °C) CITI Japan,1998<br />
Water solubility<br />
Partition coefficient n-<br />
octanol/water (log value)<br />
Henry’s law constant<br />
Appearence<br />
40 g/L (25 °C)<br />
decomposition at 80 °C<br />
CITI Japan,1998<br />
-0.52 (25 °C) CITI Japan,1998<br />
2.25 X 10 -10 atm.m 3 /mole<br />
White crystalline odorless powder<br />
HENRYWIN version<br />
1.90, Syracuse Research<br />
Co.<br />
Dutch, 1998 and<br />
Gerhartz, 1985<br />
2 GENERAL INFORMATION ON EXPOSURE<br />
2.1 Production Volumes and Use Pattern<br />
The production volume of cyanoguanidine was estimated at approximately 40,000 t/year worldwide<br />
in 2002. The chemicalis currently not manufactured in Japan. This substance is a basic chemical,<br />
and used in industry for electrical/electronic engineering, metal extraction, refining and processing<br />
of metals, paper, pulp and board, textile processing, pharmaceuticals and intermediates and<br />
approved as an indirect food additive by USFDA. This substance is also used as absorbent,<br />
adhesive, binding, coloring, electroplating, surface-active agent, and agricultural chemical<br />
(fertilizer).<br />
2.2 Environmental Exposure and Fate<br />
2.2.1 Sources of Environmental Exposure<br />
This substance has various uses and exposure to the environment is possible. This substance is<br />
directly released into the environment through its use as a fertilizer.<br />
2.2.2 Photodegradation<br />
Indirect photo-oxidation by hydroxy radicals in the atmosphere is predicted to occur with a half-life<br />
of 3.1 hrs (12-hrs day; 1.5 X 10 6 OH/cm 3 , calculated using AOPWIN version 1.90, Syracuse<br />
Research Co.).<br />
2.2.3 Stability in Water<br />
This substance is considered abiotically stable in water and not hydrolyzed regardless of pH [CITI<br />
Japan, 1998].<br />
6<br />
UNEP PUBLICATIONS