School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
N<br />
N<br />
P<br />
Fe P(C 6H 5 ) 2<br />
O<br />
P<br />
P<br />
N<br />
O O<br />
P(C 6 H 5 ) 2<br />
Quiphos<br />
BPPFA: X= (CH 3 ) 2 N<br />
Diop<br />
O<br />
O PPh 2<br />
(C 6 H 5 ) 2 P<br />
P(C 6 H 5 ) 2<br />
O PPh 2<br />
P(C 6 H 5 ) 2<br />
P(C 6 H 5 ) N<br />
2<br />
O<br />
X<br />
BPPM: X=(CH 3 ) 3 COCO<br />
PPCP<br />
Synphos<br />
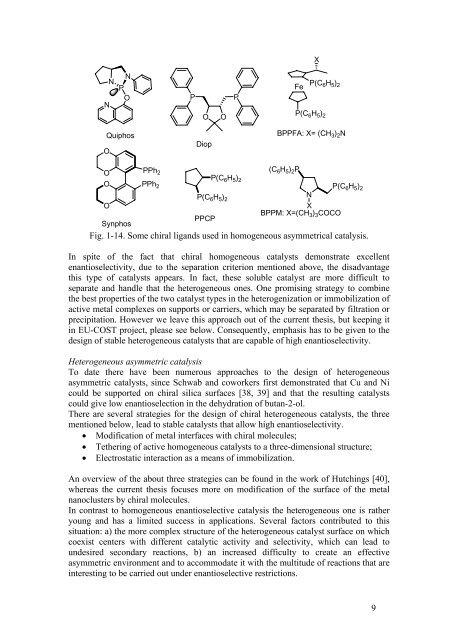
Fig. 1-14. Some chiral lig<strong>and</strong>s used in homogeneous asymmetrical catalysis.<br />
In spite <strong>of</strong> the fact that chiral homogeneous catalysts demonstrate excellent<br />
enantioselectivity, due to the separation criterion mentioned above, the disadvantage<br />
this type <strong>of</strong> catalysts appears. In fact, these soluble catalyst are more difficult to<br />
separate <strong>and</strong> h<strong>and</strong>le that the heterogeneous ones. One promising strategy to combine<br />
the best properties <strong>of</strong> the two catalyst types in the heterogenization or immobilization <strong>of</strong><br />
active metal complexes on supports or carriers, which may be separated by filtration or<br />
precipitation. However we leave this approach out <strong>of</strong> the current thesis, but keeping it<br />
in EU-COST project, please see below. Consequently, emphasis has to be given to the<br />
design <strong>of</strong> stable heterogeneous catalysts that are capable <strong>of</strong> high enantioselectivity.<br />
Heterogeneous asymmetric catalysis<br />
To date there have been numerous approaches to the design <strong>of</strong> heterogeneous<br />
asymmetric catalysts, since Schwab <strong>and</strong> coworkers first demonstrated that Cu <strong>and</strong> Ni<br />
could be supported on chiral silica surfaces [38, 39] <strong>and</strong> that the resulting catalysts<br />
could give low enantioselection in the dehydration <strong>of</strong> butan-2-ol.<br />
There are several strategies for the design <strong>of</strong> chiral heterogeneous catalysts, the three<br />
mentioned below, lead to stable catalysts that allow high enantioselectivity.<br />
• Modification <strong>of</strong> metal interfaces with chiral molecules;<br />
• Tethering <strong>of</strong> active homogeneous catalysts to a three-dimensional structure;<br />
• Electrostatic interaction as a means <strong>of</strong> immobilization.<br />
An overview <strong>of</strong> the about three strategies can be found in the work <strong>of</strong> Hutchings [40],<br />
whereas the current thesis focuses more on modification <strong>of</strong> the surface <strong>of</strong> the metal<br />
nanoclusters by chiral molecules.<br />
In contrast to homogeneous enantioselective catalysis the heterogeneous one is rather<br />
young <strong>and</strong> has a limited success in applications. Several factors contributed to this<br />
situation: a) the more complex structure <strong>of</strong> the heterogeneous catalyst surface on which<br />
coexist centers with different catalytic activity <strong>and</strong> selectivity, which can lead to<br />
undesired secondary reactions, b) an increased difficulty to create an effective<br />
asymmetric environment <strong>and</strong> to accommodate it with the multitude <strong>of</strong> reactions that are<br />
interesting to be carried out under enantioselective restrictions.<br />
X<br />
9