School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
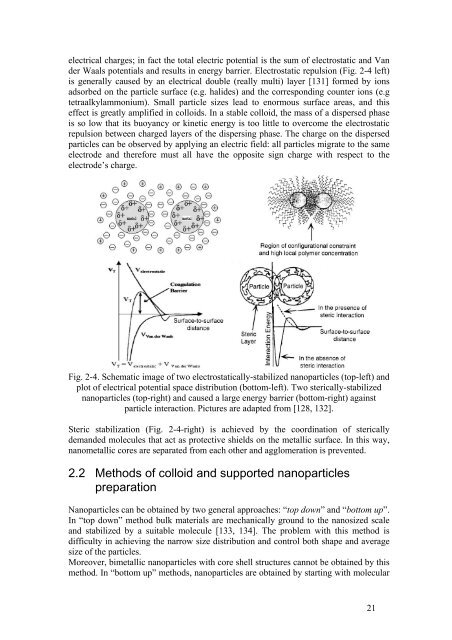
electrical charges; in fact the total electric potential is the sum <strong>of</strong> electrostatic <strong>and</strong> Van<br />
der Waals potentials <strong>and</strong> results in energy barrier. Electrostatic repulsion (Fig. 2-4 left)<br />
is generally caused by an electrical double (really multi) layer [131] formed by ions<br />
adsorbed on the particle surface (e.g. halides) <strong>and</strong> the corresponding counter ions (e.g<br />
tetraalkylammonium). Small particle sizes lead to enormous surface areas, <strong>and</strong> this<br />
effect is greatly amplified in colloids. In a stable colloid, the mass <strong>of</strong> a dispersed phase<br />
is so low that its buoyancy or kinetic energy is too little to overcome the electrostatic<br />
repulsion between charged layers <strong>of</strong> the dispersing phase. The charge on the dispersed<br />
particles can be observed by applying an electric field: all particles migrate to the same<br />
electrode <strong>and</strong> therefore must all have the opposite sign charge with respect to the<br />
electrode’s charge.<br />
Fig. 2-4. Schematic image <strong>of</strong> two electrostatically-stabilized nanoparticles (top-left) <strong>and</strong><br />
plot <strong>of</strong> electrical potential space distribution (bottom-left). Two sterically-stabilized<br />
nanoparticles (top-right) <strong>and</strong> caused a large energy barrier (bottom-right) against<br />
particle interaction. Pictures are adapted from [128, 132].<br />
Steric stabilization (Fig. 2-4-right) is achieved by the coordination <strong>of</strong> sterically<br />
dem<strong>and</strong>ed molecules that act as protective shields on the metallic surface. In this way,<br />
nanometallic cores are separated from each other <strong>and</strong> agglomeration is prevented.<br />
2.2 Methods <strong>of</strong> colloid <strong>and</strong> supported nanoparticles<br />
preparation<br />
Nanoparticles can be obtained by two general approaches: “top down” <strong>and</strong> “bottom up”.<br />
In “top down” method bulk materials are mechanically ground to the nanosized scale<br />
<strong>and</strong> stabilized by a suitable molecule [133, 134]. The problem with this method is<br />
difficulty in achieving the narrow size distribution <strong>and</strong> control both shape <strong>and</strong> average<br />
size <strong>of</strong> the particles.<br />
Moreover, bimetallic nanoparticles with core shell structures cannot be obtained by this<br />
method. In “bottom up” methods, nanoparticles are obtained by starting with molecular<br />
21