School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
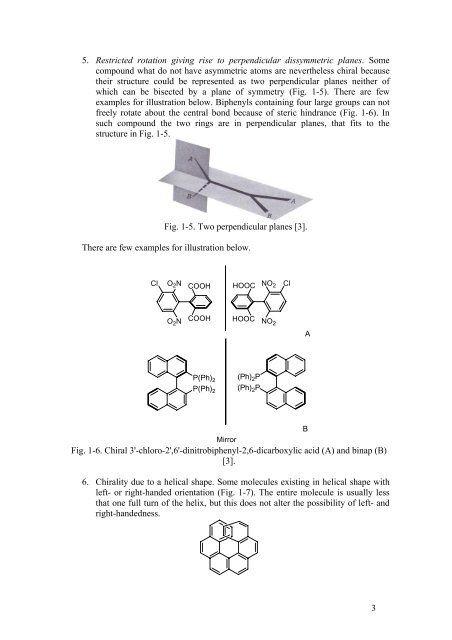
5. Restricted rotation giving rise to perpendicular dissymmetric planes. Some<br />
compound what do not have asymmetric atoms are nevertheless chiral because<br />
their structure could be represented as two perpendicular planes neither <strong>of</strong><br />
which can be bisected by a plane <strong>of</strong> symmetry (Fig. 1-5). There are few<br />
examples for illustration below. Biphenyls containing four large groups can not<br />
freely rotate about the central bond because <strong>of</strong> steric hindrance (Fig. 1-6). In<br />
such compound the two rings are in perpendicular planes, that fits to the<br />
structure in Fig. 1-5.<br />
Fig. 1-5. Two perpendicular planes [3].<br />
There are few examples for illustration below.<br />
Cl<br />
O 2 N<br />
COOH<br />
HOOC<br />
NO 2<br />
Cl<br />
O 2 N<br />
COOH<br />
HOOC<br />
NO 2<br />
A<br />
P(Ph) 2<br />
P(Ph) 2<br />
(Ph) 2 P<br />
(Ph) 2 P<br />
Mirror<br />
Fig. 1-6. Chiral 3'-chloro-2',6'-dinitrobiphenyl-2,6-dicarboxylic acid (A) <strong>and</strong> binap (B)<br />
[3].<br />
6. Chirality due to a helical shape. Some molecules existing in helical shape with<br />
left- or right-h<strong>and</strong>ed orientation (Fig. 1-7). The entire molecule is usually less<br />
that one full turn <strong>of</strong> the helix, but this does not alter the possibility <strong>of</strong> left- <strong>and</strong><br />
right-h<strong>and</strong>edness.<br />
B<br />
3