School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
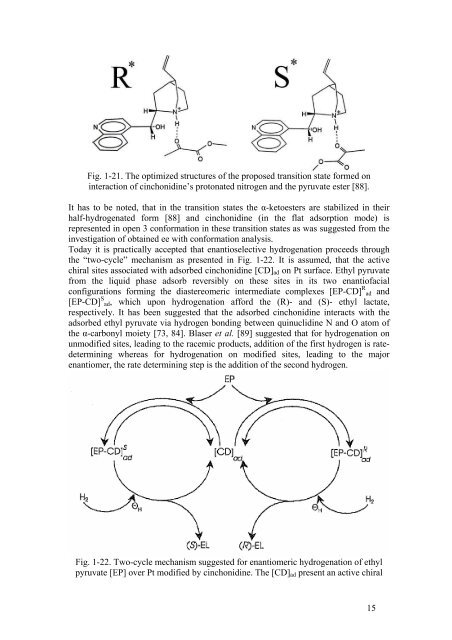
Fig. 1-21. The optimized structures <strong>of</strong> the proposed transition state formed on<br />
interaction <strong>of</strong> cinchonidine’s protonated nitrogen <strong>and</strong> the pyruvate ester [88].<br />
It has to be noted, that in the transition states the α-ketoesters are stabilized in their<br />
half-hydrogenated form [88] <strong>and</strong> cinchonidine (in the flat adsorption mode) is<br />
represented in open 3 conformation in these transition states as was suggested from the<br />
investigation <strong>of</strong> obtained ee with conformation analysis.<br />
Today it is practically accepted that enantioselective hydrogenation proceeds through<br />
the “two-cycle” mechanism as presented in Fig. 1-22. It is assumed, that the active<br />
chiral sites associated with adsorbed cinchonidine [CD] ad on Pt surface. Ethyl pyruvate<br />
from the liquid phase adsorb reversibly on these sites in its two enanti<strong>of</strong>acial<br />
configurations forming the diastereomeric intermediate complexes [EP-CD] R ad <strong>and</strong><br />
[EP-CD] S ad, which upon hydrogenation afford the (R)- <strong>and</strong> (S)- ethyl lactate,<br />
respectively. It has been suggested that the adsorbed cinchonidine interacts with the<br />
adsorbed ethyl pyruvate via hydrogen bonding between quinuclidine N <strong>and</strong> O atom <strong>of</strong><br />
the α-carbonyl moiety [73, 84]. Blaser et al. [89] suggested that for hydrogenation on<br />
unmodified sites, leading to the racemic products, addition <strong>of</strong> the first hydrogen is ratedetermining<br />
whereas for hydrogenation on modified sites, leading to the major<br />
enantiomer, the rate determining step is the addition <strong>of</strong> the second hydrogen.<br />
Fig. 1-22. Two-cycle mechanism suggested for enantiomeric hydrogenation <strong>of</strong> ethyl<br />
pyruvate [EP] over Pt modified by cinchonidine. The [CD] ad present an active chiral<br />
15