School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 1<br />
Introduction to the enantioselective<br />
catalysis<br />
1.1 General aspects: optical activity <strong>and</strong> chirality<br />
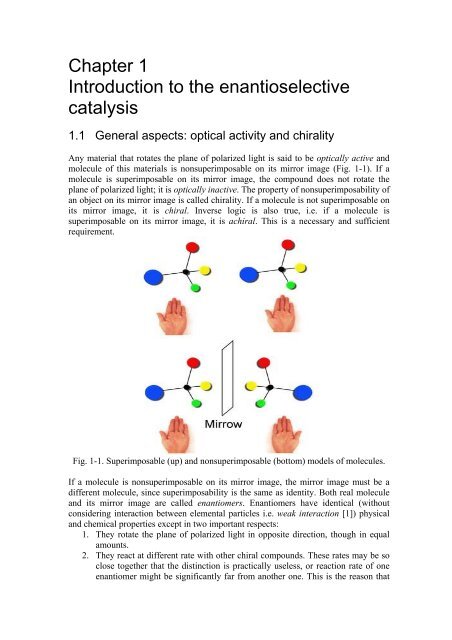
Any material that rotates the plane <strong>of</strong> polarized light is said to be optically active <strong>and</strong><br />
molecule <strong>of</strong> this materials is nonsuperimposable on its mirror image (Fig. 1-1). If a<br />
molecule is superimposable on its mirror image, the compound does not rotate the<br />
plane <strong>of</strong> polarized light; it is optically inactive. The property <strong>of</strong> nonsuperimposability <strong>of</strong><br />
an object on its mirror image is called chirality. If a molecule is not superimposable on<br />
its mirror image, it is chiral. Inverse logic is also true, i.e. if a molecule is<br />
superimposable on its mirror image, it is achiral. This is a necessary <strong>and</strong> sufficient<br />
requirement.<br />
Fig. 1-1. Superimposable (up) <strong>and</strong> nonsuperimposable (bottom) models <strong>of</strong> molecules.<br />
If a molecule is nonsuperimposable on its mirror image, the mirror image must be a<br />
different molecule, since superimposability is the same as identity. Both real molecule<br />
<strong>and</strong> its mirror image are called enantiomers. Enantiomers have identical (without<br />
considering interaction between elemental particles i.e. weak interaction [1]) physical<br />
<strong>and</strong> chemical properties except in two important respects:<br />
1. They rotate the plane <strong>of</strong> polarized light in opposite direction, though in equal<br />
amounts.<br />
2. They react at different rate with other chiral compounds. These rates may be so<br />
close together that the distinction is practically useless, or reaction rate <strong>of</strong> one<br />
enantiomer might be significantly far from another one. This is the reason that