School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
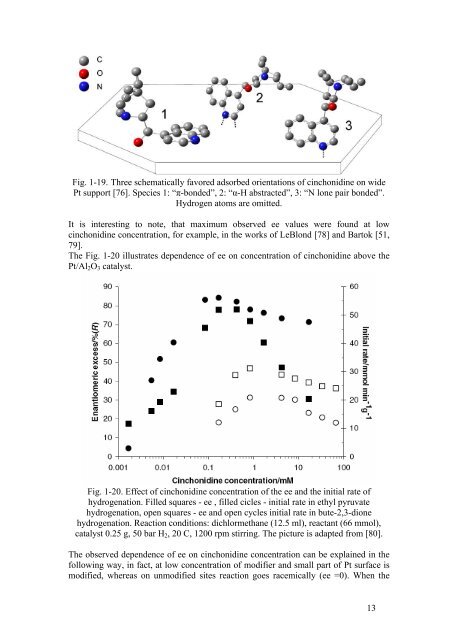
Fig. 1-19. Three schematically favored adsorbed orientations <strong>of</strong> cinchonidine on wide<br />
Pt support [76]. Species 1: “π-bonded”, 2: “α-H abstracted”, 3: “N lone pair bonded”.<br />
Hydrogen atoms are omitted.<br />
It is interesting to note, that maximum observed ee values were found at low<br />
cinchonidine concentration, for example, in the works <strong>of</strong> LeBlond [78] <strong>and</strong> Bartok [51,<br />
79].<br />
The Fig. 1-20 illustrates dependence <strong>of</strong> ee on concentration <strong>of</strong> cinchonidine above the<br />
Pt/Al 2 O 3 catalyst.<br />
Fig. 1-20. Effect <strong>of</strong> cinchonidine concentration <strong>of</strong> the ee <strong>and</strong> the initial rate <strong>of</strong><br />
hydrogenation. Filled squares - ee , filled cicles - initial rate in ethyl pyruvate<br />
hydrogenation, open squares - ee <strong>and</strong> open cycles initial rate in bute-2,3-dione<br />
hydrogenation. Reaction conditions: dichlormethane (12.5 ml), reactant (66 mmol),<br />
catalyst 0.25 g, 50 bar H 2 , 20 C, 1200 rpm stirring. The picture is adapted from [80].<br />
The observed dependence <strong>of</strong> ee on cinchonidine concentration can be explained in the<br />
following way, in fact, at low concentration <strong>of</strong> modifier <strong>and</strong> small part <strong>of</strong> Pt surface is<br />
modified, whereas on unmodified sites reaction goes racemically (ee =0). When the<br />
13