Brugia Malayi - Clark Science Center - Smith College
Brugia Malayi - Clark Science Center - Smith College
Brugia Malayi - Clark Science Center - Smith College
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The Stabilizing Effect of Metalloporphyrins on Myoglobin<br />
Metasebia Aberra<br />
30<br />
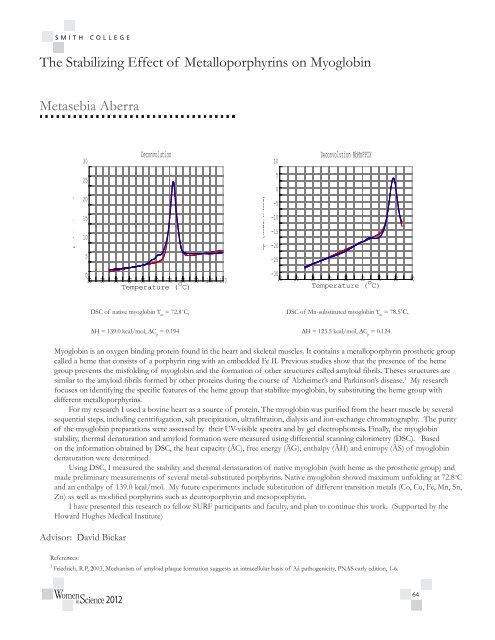
Deconvolution<br />
10<br />
Deconvolution MbMnPPIX<br />
Cp (kcal/K mol)<br />
25<br />
20<br />
15<br />
10<br />
5<br />
Cp (kcal/K mol)<br />
5<br />
0<br />
-5<br />
-10<br />
-15<br />
-20<br />
-25<br />
0<br />
10 20 30 40 50 60 70<br />
Temperature ( o 80 90 100 110<br />
C)<br />
-30<br />
10 20 30 40 50 60<br />
Temperature ( o 70 80 90<br />
C)<br />
DSC of native myoglobin T m<br />
= 72.8˚C,<br />
DSC of Mn-substituted myoglobin T m<br />
= 78.5˚C,<br />
∆H = 139.0 kcal/mol, ∆C p<br />
= 0.194 ∆H = 123.5 kcal/mol, ∆C p<br />
= 0.124<br />
Myoglobin is an oxygen binding protein found in the heart and skeletal muscles. It contains a metalloporphyrin prosthetic group<br />
called a heme that consists of a porphyrin ring with an embedded Fe II. Previous studies show that the presence of the heme<br />
group prevents the misfolding of myoglobin and the formation of other structures called amyloid fibrils. Theses structures are<br />
similar to the amyloid fibrils formed by other proteins during the course of Alzheimer’s and Parkinson’s disease. 1 My research<br />
focuses on identifying the specific features of the heme group that stabilize myoglobin, by substituting the heme group with<br />
different metalloporphyrins.<br />
For my research I used a bovine heart as a source of protein. The myoglobin was purified from the heart muscle by several<br />
sequential steps, including centrifugation, salt precipitation, ultrafiltration, dialysis and ion-exchange chromatography. The purity<br />
of the myoglobin preparations were assessed by their UV-visible spectra and by gel electrophoresis. Finally, the myoglobin<br />
stability, thermal denaturation and amyloid formation were measured using differential scanning calorimetry (DSC). Based<br />
on the information obtained by DSC, the heat capacity (ÄC), free energy (ÄG), enthalpy (ÄH) and entropy (ÄS) of myoglobin<br />
denaturation were determined.<br />
Using DSC, I measured the stability and thermal denaturation of native myoglobin (with heme as the prosthetic group) and<br />
made preliminary measurements of several metal-substituted porphyrins. Native myoglobin showed maximum unfolding at 72.8 o C<br />
and an enthalpy of 139.0 kcal/mol. My future experiments include substitution of different transition metals (Co, Cu, Fe, Mn, Sn,<br />
Zn) as well as modified porphyrins such as deutroporphyrin and mesoporphyrin.<br />
I have presented this research to fellow SURF participants and faculty, and plan to continue this work. (Supported by the<br />
Howard Hughes Medical Institute)<br />
Advisor: David Bickar<br />
References:<br />
1<br />
Friedrich, R.P, 2003, Mechanism of amyloid plaque formation suggests an intracellular basis of Aâ pathogenicity, PNAS early edition, 1-6.<br />
2012<br />
64