(RASFF) Annual Report 2009 - European Commission - Europa
(RASFF) Annual Report 2009 - European Commission - Europa
(RASFF) Annual Report 2009 - European Commission - Europa
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Annual</strong> <strong>Report</strong> <strong>2009</strong><br />
dose 13 for triazophos. An intake above the acute reference dose could lead to<br />
acute poisoning effects. Consumption data are used to calculate the short<br />
term intake. For okra however, consumption data do not exist in Europe. It is<br />
therefore common practice to use intake data of a comparable vegetable, in<br />
this case e. g. green beans. At the levels found, the intake calculated exceeded<br />
the acute reference dose considerably.<br />
Another problem with the enforcement of safe pesticide residue levels in<br />
food on the market is the short shelf life of fresh fruit and vegetables. When<br />
samples are taken from produce, usually the produce is not detained pending<br />
the results. When the results are available, the produce is often already sold<br />
and consumed.<br />
Market notifications are only transmitted if the levels found present a risk to<br />
the consumer. A calculation is made comparing short term intake with acute<br />
reference dose. However, when the product is stopped at the EU border and<br />
sampled for pesticide residues, it remains blocked until results are available. If<br />
the results are unfavourable, meaning that one or more residues were found<br />
above the MRL, then the consignment is destroyed or redispatched according<br />
to the decision of the competent authority and a border rejection notification<br />
is transmitted.<br />
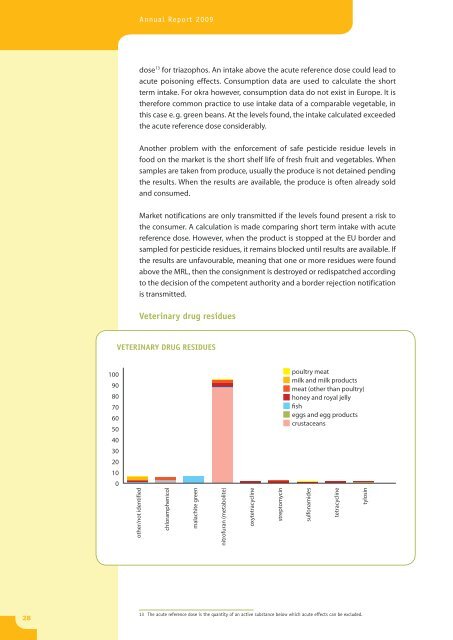
Veterinary drug residues<br />
VETERINARY DRUG RESIDUES<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
poultry meat<br />
milk and milk products<br />
meat (other than poultry)<br />
honey and royal jelly<br />
fish<br />
eggs and egg products<br />
crustaceans<br />
other/not identified<br />
chloramphenicol<br />
malachite green<br />
nitrofuran (metabolite)<br />
oxytetracycline<br />
streptomycin<br />
sulfonamides<br />
tetracycline<br />
tylosin<br />
28<br />
13 The acute reference dose is the quantity of an active substance below which acute effects can be excluded.