The Compleat Distiller
The Compleat Distiller
The Compleat Distiller
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
THE COMPLEAT DISTILLER 21<br />
An illustration of vapor pressure<br />
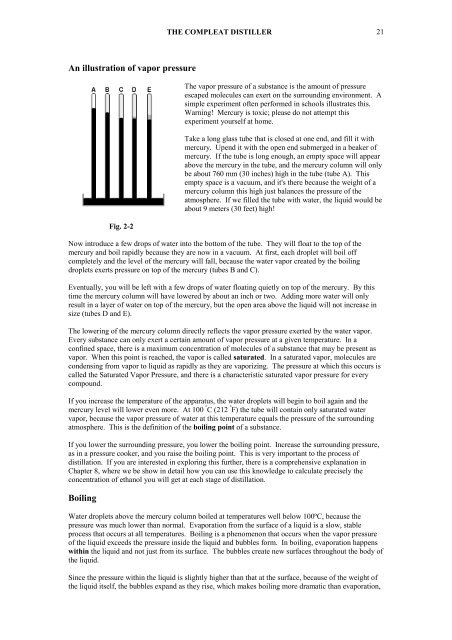
Fig. 2-2<br />
<strong>The</strong> vapor pressure of a substance is the amount of pressure<br />
escaped molecules can exert on the surrounding environment. A<br />
simple experiment often performed in schools illustrates this.<br />
Warning! Mercury is toxic; please do not attempt this<br />
experiment yourself at home.<br />
Take a long glass tube that is closed at one end, and fill it with<br />
mercury. Upend it with the open end submerged in a beaker of<br />
mercury. If the tube is long enough, an empty space will appear<br />
above the mercury in the tube, and the mercury column will only<br />
be about 760 mm (30 inches) high in the tube (tube A). This<br />
empty space is a vacuum, and it's there because the weight of a<br />
mercury column this high just balances the pressure of the<br />
atmosphere. If we filled the tube with water, the liquid would be<br />
about 9 meters (30 feet) high!<br />
Now introduce a few drops of water into the bottom of the tube. <strong>The</strong>y will float to the top of the<br />
mercury and boil rapidly because they are now in a vacuum. At first, each droplet will boil off<br />
completely and the level of the mercury will fall, because the water vapor created by the boiling<br />
droplets exerts pressure on top of the mercury (tubes B and C).<br />
Eventually, you will be left with a few drops of water floating quietly on top of the mercury. By this<br />
time the mercury column will have lowered by about an inch or two. Adding more water will only<br />
result in a layer of water on top of the mercury, but the open area above the liquid will not increase in<br />
size (tubes D and E).<br />
<strong>The</strong> lowering of the mercury column directly reflects the vapor pressure exerted by the water vapor.<br />
Every substance can only exert a certain amount of vapor pressure at a given temperature. In a<br />
confined space, there is a maximum concentration of molecules of a substance that may be present as<br />
vapor. When this point is reached, the vapor is called saturated. In a saturated vapor, molecules are<br />
condensing from vapor to liquid as rapidly as they are vaporizing. <strong>The</strong> pressure at which this occurs is<br />
called the Saturated Vapor Pressure, and there is a characteristic saturated vapor pressure for every<br />
compound.<br />
If you increase the temperature of the apparatus, the water droplets will begin to boil again and the<br />
mercury level will lower even more. At 100 º C (212 º F) the tube will contain only saturated water<br />
vapor, because the vapor pressure of water at this temperature equals the pressure of the surrounding<br />
atmosphere. This is the definition of the boiling point of a substance.<br />
If you lower the surrounding pressure, you lower the boiling point. Increase the surrounding pressure,<br />
as in a pressure cooker, and you raise the boiling point. This is very important to the process of<br />
distillation. If you are interested in exploring this further, there is a comprehensive explanation in<br />
Chapter 8, where we be show in detail how you can use this knowledge to calculate precisely the<br />
concentration of ethanol you will get at each stage of distillation.<br />
Boiling<br />
Water droplets above the mercury column boiled at temperatures well below 100ºC, because the<br />
pressure was much lower than normal. Evaporation from the surface of a liquid is a slow, stable<br />
process that occurs at all temperatures. Boiling is a phenomenon that occurs when the vapor pressure<br />
of the liquid exceeds the pressure inside the liquid and bubbles form. In boiling, evaporation happens<br />
within the liquid and not just from its surface. <strong>The</strong> bubbles create new surfaces throughout the body of<br />
the liquid.<br />
Since the pressure within the liquid is slightly higher than that at the surface, because of the weight of<br />
the liquid itself, the bubbles expand as they rise, which makes boiling more dramatic than evaporation,