A guide for the preparation and use of buffers in biological systems
A guide for the preparation and use of buffers in biological systems
A guide for the preparation and use of buffers in biological systems
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
10<br />
987<br />
6<br />
5<br />
4<br />
3<br />
2<br />
[A - ]/[HA]<br />
1<br />
0.9<br />
0.8<br />
0.7<br />
0.6<br />
0.5<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
0.1 -0.8 -0.6 -0.4 -0.2 pK a 0.2 0.4 0.6 0.8 1.0<br />
∆ pH from pK a<br />
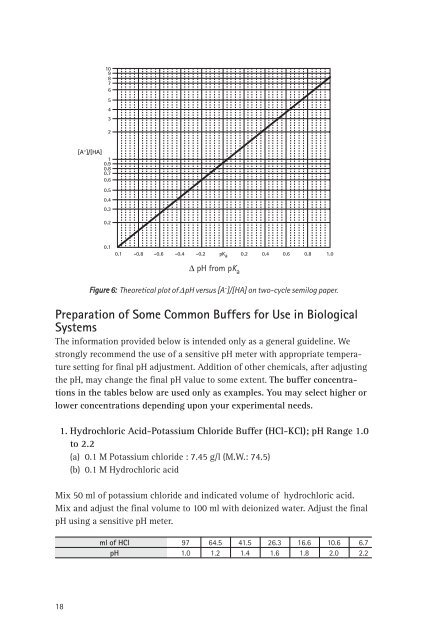
Figure 6: Theoretical plot <strong>of</strong> DpH versus [A - ]/[HA] on two-cycle semilog paper.<br />
Preparation <strong>of</strong> Some Common Buffers <strong>for</strong> Use <strong>in</strong> Biological<br />
Systems<br />
The <strong>in</strong><strong>for</strong>mation provided below is <strong>in</strong>tended only as a general <strong>guide</strong>l<strong>in</strong>e. We<br />
strongly recommend <strong>the</strong> <strong>use</strong> <strong>of</strong> a sensitive pH meter with appropriate temperature<br />
sett<strong>in</strong>g <strong>for</strong> f<strong>in</strong>al pH adjustment. Addition <strong>of</strong> o<strong>the</strong>r chemicals, after adjust<strong>in</strong>g<br />
<strong>the</strong> pH, may change <strong>the</strong> f<strong>in</strong>al pH value to some extent. The buffer concentrations<br />
<strong>in</strong> <strong>the</strong> tables below are <strong>use</strong>d only as examples. You may select higher or<br />
lower concentrations depend<strong>in</strong>g upon your experimental needs.<br />
1. Hydrochloric Acid-Potassium Chloride Buffer (HCl-KCl); pH Range 1.0<br />
to 2.2<br />
(a) 0.1 M Potassium chloride : 7.45 g/l (M.W.: 74.5)<br />
(b) 0.1 M Hydrochloric acid<br />
Mix 50 ml <strong>of</strong> potassium chloride <strong>and</strong> <strong>in</strong>dicated volume <strong>of</strong> hydrochloric acid.<br />
Mix <strong>and</strong> adjust <strong>the</strong> f<strong>in</strong>al volume to 100 ml with deionized water. Adjust <strong>the</strong> f<strong>in</strong>al<br />
pH us<strong>in</strong>g a sensitive pH meter.<br />
ml <strong>of</strong> HCl 97 64.5 41.5 26.3 16.6 10.6 6.7<br />
pH 1.0 1.2 1.4 1.6 1.8 2.0 2.2<br />
18