A guide for the preparation and use of buffers in biological systems
A guide for the preparation and use of buffers in biological systems
A guide for the preparation and use of buffers in biological systems
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
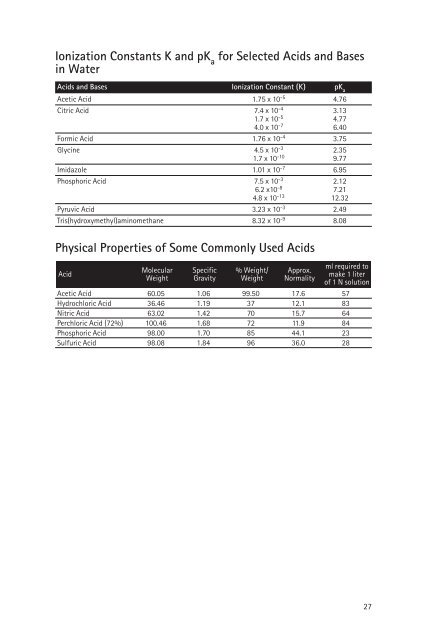
Ionization Constants K <strong>and</strong> pK a<br />
<strong>for</strong> Selected Acids <strong>and</strong> Bases<br />
<strong>in</strong> Water<br />
Acids <strong>and</strong> Bases Ionization Constant (K) pK a<br />
Acetic Acid 1.75 x 10 -5 4.76<br />
Citric Acid 7.4 x 10 -4 3.13<br />
1.7 x 10 -5 4.77<br />
4.0 x 10 -7 6.40<br />
Formic Acid 1.76 x 10 -4 3.75<br />
Glyc<strong>in</strong>e 4.5 x 10 -3 2.35<br />
1.7 x 10 -10 9.77<br />
Imidazole 1.01 x 10 -7 6.95<br />
Phosphoric Acid 7.5 x 10 -3 2.12<br />
6.2 x10 -8 7.21<br />
4.8 x 10 -13 12.32<br />
Pyruvic Acid 3.23 x 10 -3 2.49<br />
Tris(hydroxymethyl)am<strong>in</strong>omethane 8.32 x 10 -9 8.08<br />
Physical Properties <strong>of</strong> Some Commonly Used Acids<br />
Molecular Specific % Weight/ Approx.<br />
ml required to<br />
Acid<br />
Weight Gravity Weight Normality<br />
make 1 liter<br />
<strong>of</strong> 1 N solution<br />
Acetic Acid 60.05 1.06 99.50 17.6 57<br />
Hydrochloric Acid 36.46 1.19 37 12.1 83<br />
Nitric Acid 63.02 1.42 70 15.7 64<br />
Perchloric Acid (72%) 100.46 1.68 72 11.9 84<br />
Phosphoric Acid 98.00 1.70 85 44.1 23<br />
Sulfuric Acid 98.08 1.84 96 36.0 28<br />
27