Chapter 11 Chemical Bonds: The Formation of Compounds from ...
Chapter 11 Chemical Bonds: The Formation of Compounds from ...
Chapter 11 Chemical Bonds: The Formation of Compounds from ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
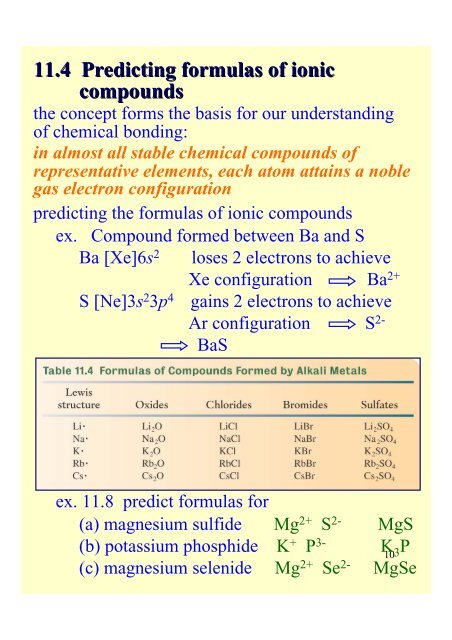
<strong>11</strong>.4 Predicting formulas <strong>of</strong> ionic<br />
compounds<br />
the concept forms the basis for our understanding<br />
<strong>of</strong> chemical bonding:<br />
in almost all stable chemical compounds <strong>of</strong><br />
representative elements, each atom attains a noble<br />
gas electron configuration<br />
predicting the formulas <strong>of</strong> ionic compounds<br />
ex. Compound formed between Ba and S<br />
Ba [Xe]6s 2<br />
S [Ne]3s 2 3p 4<br />
loses 2 electrons to achieve<br />
Xe configuration Ba 2+<br />
gains 2 electrons to achieve<br />
Ar configuration S 2-<br />
BaS<br />
ex. <strong>11</strong>.8 predict formulas for<br />
(a) magnesium sulfide Mg 2+ S 2- MgS<br />
(b) potassium phosphide K + P 3- K10<br />
3 P<br />
(c) magnesium selenide Mg 2+ Se 2- MgSe











![Hetero [6+3] Cycloaddition of Fulvenes with N-Alkylidene Glycine ...](https://img.yumpu.com/35423358/1/190x245/hetero-6-3-cycloaddition-of-fulvenes-with-n-alkylidene-glycine-.jpg?quality=85)



