Chapter 11 Chemical Bonds: The Formation of Compounds from ...
Chapter 11 Chemical Bonds: The Formation of Compounds from ...
Chapter 11 Chemical Bonds: The Formation of Compounds from ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
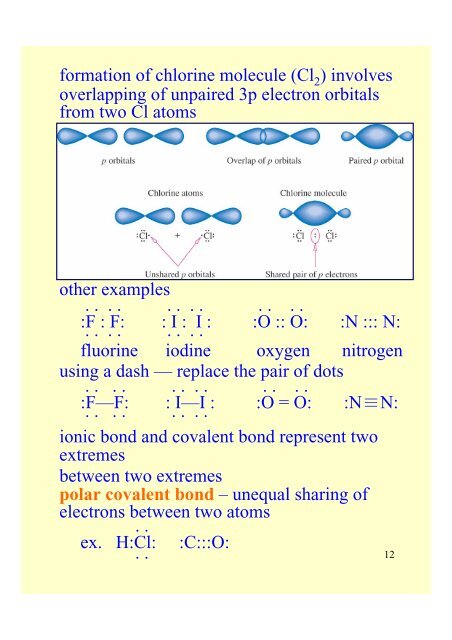
formation <strong>of</strong> chlorine molecule (Cl 2 ) involves<br />
overlapping <strong>of</strong> unpaired 3p electron orbitals<br />
<strong>from</strong> two Cl atoms<br />
other examples<br />
. . . . . . . . . . . .<br />
:F : F: : I : I : :O :: O: :N ::: N:<br />
·· ·· ·· ··<br />
fluorine iodine oxygen nitrogen<br />
using a dash — replace the pair <strong>of</strong> dots<br />
. . . . . . . . . . . .<br />
:F—F: : I—I : :O = O: :N≣N:<br />
·· ·· ·· ··<br />
ionic bond and covalent bond represent two<br />
extremes<br />
between two extremes<br />
polar covalent bond – unequal sharing <strong>of</strong><br />
electrons between two atoms<br />
. .<br />
ex. H:Cl: :C:::O:<br />
12<br />
··











![Hetero [6+3] Cycloaddition of Fulvenes with N-Alkylidene Glycine ...](https://img.yumpu.com/35423358/1/190x245/hetero-6-3-cycloaddition-of-fulvenes-with-n-alkylidene-glycine-.jpg?quality=85)



