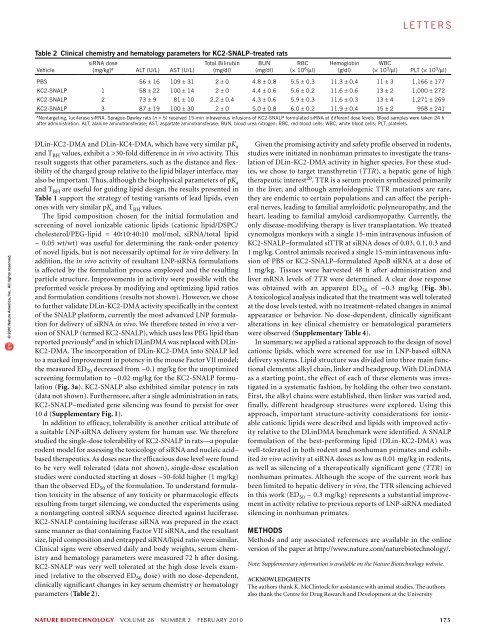
l e t t e r s© 2010 Nature America, Inc. All rights reserved.Table 1 Biophysical parameters and in vivo activities ofLNPs containing novel lipidsCationic lipidApparentlipid pK aaL II to H II phase transition In vivo ED 50temperature (°C) b (mg/kg)DLinDMA 6.8 ± 0.10 27 ~1DLinDAP 6.2 ± 0.05 26 40–50DLin-K-DMA 5.9 ± 0.03 19 ~0.4DLin-KC2-DMA 6.7 ± 0.08 20 ~0.1DLin-KC3-DMA 7.2 ± 0.05 18 ~0.6DLin-KC4-DMA 7.3 ± 0.07 18 >3a pK a values ± s.d. (n = 3 to 9). b L II to H II phase transition was measured at pH 4.8 in equimolarmixtures with DSPS, using differential scanning calorimetric, repeat scans reproducibleto within 0.1 °C.affect both the pK a of the amine headgroup as well as the distanceand flexibility of the charge presentation relative to the lipid bilayerinterface. Inserting a single additional methylene group into the headgroup(DLin-KC2-DMA) produced a dramatic increase in potencyrelative to DLin-K-DMA. The ED 50 for this lipid was ~0.1 mg/kg,making it fourfold more potent than DLin-K-DMA and tenfold morepotent than the DLinDMA benchmark when compared head-to-headin the Factor VII model (Fig. 2a). Further extension of the tether withadditional methylene groups, however, substantially decreased activity,with an ED 50 of ~0.6 mg/kg for DLin-KC3-DMA and >3 mg/kgfor DLin-KC4-DMA (Fig. 2b).As changes in lipid design and chemistry may affect the pharmacokinetics,target tissue accumulation and intracellular delivery of LNPformulations, we investigated the relative importance of these parameterson LNP activity at an early stage in this research program.Several of the novel lipids were incorporated into LNP-siRNA formulationscontaining cyanine dye (Cy3)-labeled siRNA. Plasma,liver and spleen levels of siRNA were determined at 0.5 and 3 h afterinjection at siRNA doses of 5 mg/kg, and the results are presented inSupplementary Table 3. In general, formulations that were the mostactive in the mouse Factor VII screens achieved the highest liver levelsof siRNA at 0.5 h; however, delivery of siRNA to the target tissue wasnot the primary factor responsible for activity. This is supported bythe observations that most formulations accumulated in the liver andspleen quite quickly and that some formulations with similar liver levelsof siRNA had large differences in activity. Moreover, plasma pharmacokineticsalone did not predict activity. For example, althoughDLin-KC2-DMA and DLinDMA had virtually indistinguishableblood pharmacokinetic profiles in mice (data not shown), the activityof DLin-KC2-DMA in LNPs is approximately tenfold greater than thesame formulation with DLinDMA. Taken together, these results ledus to conclude that rapid target tissue accumulation was important,but not sufficient, for activity. Moreover, other parameters were morecritical for maximizing the activity of LNP-siRNA formulations.Two important parameters underlying lipid design for SNALPmediateddelivery are the pK a of the ionizable cationic lipid and theabilities of these lipids, when protonated, to induce a nonbilayer(hexagonal H II ) phase structure when mixed with anionic lipids.The pK a of the ionizable cationic lipid determines the surfacecharge on the LNP under different pH conditions. The charge stateat physiologic pH (e.g., in circulation) can influence plasma proteinadsorption, blood clearance and tissue distribution behavior 16 ,whereas the charge state at acidic pH (e.g., in endosomes) can influencethe ability of the LNP to combine with endogenous anioniclipids to form endosomolytic nonbilayer structures 9 . Consequently,the ability of these lipids to induce H II phase structure in mixtureswith anionic lipids is a measure of their bilayer-destabilizing capacityand relative endosomolytic potential.The fluorescent probe 2-(p-toluidino)-6-napthalene sulfonicacid (TNS), which exhibits increased fluorescence in a hydrophobicenvironment, can be used to assess surface charge on lipid bilayers.Titrations of surface charge as a function of pH can then be usedto determine the apparent pK a of the lipid in the bilayer (hereafterreferred to as pK a ) of constituent lipids 17 . Using this approach, thepK a values for LNPs containing DLinDAP, DLinDMA, DLin-K-DMA,DLin-KC2-DMA, DLin-KC3-DMA and DLin-KC4-DMA were determined(Table 1). The relative ability of the protonated form of theionizable cationic lipids to induce H II phase structure in anionic lipidswas ascertained by measuring the bilayer-to-hexagonal H II transitiontemperature (T BH ) in equimolar mixtures with distearoylphosphatidylserine(DSPS) at pH 4.8, using 31 P NMR 18 and differential scanningcalorimetric analyses 19 . Both techniques gave similar results.The data presented in Table 1 indicate that the highly active lipidDLin-KC2-DMA has pK a and T BH values that are theoretically favorablefor use in siRNA delivery systems. The pK a of 6.4 indicatesthat LNPs based on DLin-KC2-DMA have limited surface charge incirculation, but will become positively charged in endosomes. Further,the T BH for DLin-KC2-DMA is 7 °C lower than that for DLinDMA,suggesting that this lipid has improved capacity for destabilizingbilayers. However, the data also demonstrate that pK a and T BH donot fully account for the in vivo activity of lipids used in LNPs. Forexample, although DLin-KC3-DMA and DLin-KC4-DMA haveidentical pK a and T BH values, DLin-KC4-DMA requires a more thanfivefold higher dose to achieve the same activity in vivo. Moreover,Figure 3 Efficacy of KC2-SNALP in rodentsand nonhuman primates. (a) Improvedefficacy of KC2-SNALP relative to theinitial screening formulation tested inmice. The in vivo efficacy of KC2-SNALP() was compared to that of the unoptimizedDLin-KC2-DMA screening (that is, PFV)formulation ( • ) in the mouse Factor VII model.Data points are expressed as a percentageof PBS control animals and represent groupmean (n = 5) ± s.d. (b) Efficacy of KC2-SNALPin nonhuman primates. Cynomolgus monkeys(n = 3 per group) received a total dose of either0.03, 0.1, 0.3 or 1 mg/kg siTTR, or 1 mg/kgsiApoB formulated in KC2-SNALP or PBSas 15-min intravenous infusions (5 ml/kg)aRelative serum factor VII protein (%)1201008060402000.001 0.01 0.11 10Factor VII siRNA dose (mg/kg)bRelative liver TTR/GAPDH mRNA levels1.41.21.00.80.60.40.20PBS1mg/kgsiApoB0.03mg/kg**0.1 0.3mg/kg mg/kgsiTTRthrough the cephalic vein. Animals were euthanized 48 h after administration. TTR mRNA levels relative to GAPDH mRNA levels were determined inliver samples. Data points represent group mean ± s.d. *, P < 0.05; **, P < 0.005.**1mg/kg174 VOLUME 28 NUMBER 2 FEBRUARY 2010 nature biotechnology
l e t t e r sTable 2 Clinical chemistry and hematology parameters for KC2-SNALP–treated ratsVehiclesiRNA dose(mg/kg) a ALT (U/L) AST (U/L)Total Bilirubin(mg/dl)BUN(mg/dl)RBC(× 10 6 /µl)Hemoglobin(g/dl)WBC(× 10 3 /µl) PLT (× 10 3 /µl)PBS 56 ± 16 109 ± 31 2 ± 0 4.8 ± 0.8 5.5 ± 0.3 11.3 ± 0.4 11 ± 3 1,166 ± 177KC2-SNALP 1 58 ± 22 100 ± 14 2 ± 0 4.4 ± 0.6 5.6 ± 0.2 11.6 ± 0.6 13 ± 2 1,000 ± 272KC2-SNALP 2 73 ± 9 81 ± 10 2.2 ± 0.4 4.3 ± 0.6 5.9 ± 0.3 11.6 ± 0.3 13 ± 4 1,271 ± 269KC2-SNALP 3 87 ± 19 100 ± 30 2 ± 0 5.0 ± 0.8 6.0 ± 0.2 11.9 ± 0.4 15 ± 2 958 ± 241a Nontargeting, luciferase siRNA. Sprague-Dawley rats (n = 5) received 15-min intravenous infusions of KC2-SNALP formulated siRNA at different dose levels. Blood samples were taken 24 hafter administration. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; RBC, red blood cells; WBC, white blood cells; PLT, platelets.© 2010 Nature America, Inc. All rights reserved.DLin-KC2-DMA and DLin-KC4-DMA, which have very similar pK aand T BH values, exhibit a >30-fold difference in in vivo activity. Thisresult suggests that other parameters, such as the distance and flexibilityof the charged group relative to the lipid bilayer interface, mayalso be important. Thus, although the biophysical parameters of pK aand T BH are useful for guiding lipid design, the results presented inTable 1 support the strategy of testing variants of lead lipids, evenones with very similar pK a and T BH values.The lipid composition chosen for the initial formulation andscreening of novel ionizable cationic lipids (cationic lipid/DSPC/cholesterol/PEG-lipid = 40:10:40:10 mol/mol, siRNA/total lipid~ 0.05 wt/wt) was useful for determining the rank-order potencyof novel lipids, but is not necessarily optimal for in vivo delivery. Inaddition, the in vivo activity of resultant LNP-siRNA formulationsis affected by the formulation process employed and the resultingparticle structure. Improvements in activity were possible with thepreformed vesicle process by modifying and optimizing lipid ratiosand formulation conditions (results not shown). However, we choseto further validate DLin-KC2-DMA activity specifically in the contextof the SNALP platform, currently the most advanced LNP formulationfor delivery of siRNA in vivo. We therefore tested in vivo a versionof SNALP (termed KC2-SNALP), which uses less PEG lipid thanreported previously 6 and in which DLinDMA was replaced with DLin-KC2-DMA. The incorporation of DLin-KC2-DMA into SNALP ledto a marked improvement in potency in the mouse Factor VII model;the measured ED 50 decreased from ~0.1 mg/kg for the unoptimizedscreening formulation to ~0.02 mg/kg for the KC2-SNALP formulation(Fig. 3a). KC2-SNALP also exhibited similar potency in rats(data not shown). Furthermore, after a single administration in rats,KC2-SNALP–mediated gene silencing was found to persist for over10 d (Supplementary Fig. 1).In addition to efficacy, tolerability is another critical attribute ofa suitable LNP-siRNA delivery system for human use. We thereforestudied the single-dose tolerability of KC2-SNALP in rats—a popularrodent model for assessing the toxicology of siRNA and nucleic acid–based therapeutics. As doses near the efficacious dose level were foundto be very well tolerated (data not shown), single-dose escalationstudies were conducted starting at doses ~50-fold higher (1 mg/kg)than the observed ED 50 of the formulation. To understand formulationtoxicity in the absence of any toxicity or pharmacologic effectsresulting from target silencing, we conducted the experiments usinga nontargeting control siRNA sequence directed against luciferase.KC2-SNALP containing luciferase siRNA was prepared in the exactsame manner as that containing Factor VII siRNA, and the resultantsize, lipid composition and entrapped siRNA/lipid ratio were similar.Clinical signs were observed daily and body weights, serum chemistryand hematology parameters were measured 72 h after dosing.KC2-SNALP was very well tolerated at the high dose levels examined(relative to the observed ED 50 dose) with no dose-dependent,clinically significant changes in key serum chemistry or hematologyparameters (Table 2).Given the promising activity and safety profile observed in rodents,studies were initiated in nonhuman primates to investigate the translationof DLin-KC2-DMA activity in higher species. For these studies,we chose to target transthyretin (TTR), a hepatic gene of hightherapeutic interest 20 . TTR is a serum protein synthesized primarilyin the liver, and although amyloidogenic TTR mutations are rare,they are endemic to certain populations and can affect the peripheralnerves, leading to familial amyloidotic polyneuropathy, and theheart, leading to familial amyloid cardiomyopathy. Currently, theonly disease-modifying therapy is liver transplantation. We treatedcynomolgus monkeys with a single 15-min intravenous infusion ofKC2-SNALP–formulated siTTR at siRNA doses of 0.03, 0.1, 0.3 and1 mg/kg. Control animals received a single 15-min intravenous infusionof PBS or KC2-SNALP–formulated ApoB siRNA at a dose of1 mg/kg. Tissues were harvested 48 h after administration andliver mRNA levels of TTR were determined. A clear dose responsewas obtained with an apparent ED 50 of ~0.3 mg/kg (Fig. 3b).A toxicological analysis indicated that the treatment was well toleratedat the dose levels tested, with no treatment-related changes in animalappearance or behavior. No dose-dependent, clinically significantalterations in key clinical chemistry or hematological parameterswere observed (Supplementary Table 4).In summary, we applied a rational approach to the design of novelcationic lipids, which were screened for use in LNP-based siRNAdelivery systems. Lipid structure was divided into three main functionalelements: alkyl chain, linker and headgroup. With DLinDMAas a starting point, the effect of each of these elements was investigatedin a systematic fashion, by holding the other two constant.First, the alkyl chains were established, then linker was varied and,finally, different headgroup structures were explored. Using thisapproach, important structure-activity considerations for ionizablecationic lipids were described and lipids with improved activityrelative to the DLinDMA benchmark were identified. A SNALPformulation of the best-performing lipid (DLin-KC2-DMA) waswell-tolerated in both rodent and nonhuman primates and exhibitedin vivo activity at siRNA doses as low as 0.01 mg/kg in rodents,as well as silencing of a therapeutically significant gene (TTR) innonhuman primates. Although the scope of the current work hasbeen limited to hepatic delivery in vivo, the TTR silencing achievedin this work (ED 50 ~ 0.3 mg/kg) represents a substantial improvementin activity relative to previous reports of LNP-siRNA mediatedsilencing in nonhuman primates.MethodsMethods and any associated references are available in the onlineversion of the paper at http://www.nature.com/naturebiotechnology/.Note: Supplementary information is available on the Nature Biotechnology website.AcknowledgmentsThe authors thank K. McClintock for assistance with animal studies. The authorsalso thank the Centre for Drug Research and Development at the Universitynature biotechnology VOLUME 28 NUMBER 2 FEBRUARY 2010 175
- Page 3 and 4:
volume 28 number 2 february 2010COM
- Page 5 and 6:
in this issue© 2010 Nature America
- Page 7 and 8:
© 2010 Nature America, Inc. All ri
- Page 10 and 11:
NEWS© 2010 Nature America, Inc. Al
- Page 12 and 13:
NEWS© 2010 Nature America, Inc. Al
- Page 14 and 15:
NEWS© 2010 Nature America, Inc. Al
- Page 16 and 17:
© 2010 Nature America, Inc. All ri
- Page 18 and 19:
© 2010 Nature America, Inc. All ri
- Page 20 and 21:
© 2010 Nature America, Inc. All ri
- Page 22 and 23:
NEWS feature© 2010 Nature America,
- Page 24 and 25:
uilding a businessComing to termsDa
- Page 26 and 27:
uilding a business© 2010 Nature Am
- Page 28 and 29:
correspondence© 2010 Nature Americ
- Page 30 and 31:
correspondence© 2010 Nature Americ
- Page 32 and 33: correspondence© 2010 Nature Americ
- Page 34 and 35: correspondence© 2010 Nature Americ
- Page 36 and 37: case studyNever againcommentaryChri
- Page 38 and 39: COMMENTARY© 2010 Nature America, I
- Page 40 and 41: COMMENTARY© 2010 Nature America, I
- Page 42 and 43: patents© 2010 Nature America, Inc.
- Page 44 and 45: patents© 2010 Nature America, Inc.
- Page 46 and 47: news and viewsChIPs and regulatory
- Page 48 and 49: news and viewsFrom genomics to crop
- Page 50 and 51: news and views© 2010 Nature Americ
- Page 52 and 53: news and views© 2010 Nature Americ
- Page 54 and 55: e s o u r c eRational association o
- Page 56 and 57: e s o u r c e© 2010 Nature America
- Page 58 and 59: e s o u r c e© 2010 Nature America
- Page 60 and 61: e s o u r c e© 2010 Nature America
- Page 62 and 63: © 2010 Nature America, Inc. All ri
- Page 64 and 65: B r i e f c o m m u n i c at i o n
- Page 66 and 67: i e f c o m m u n i c at i o n sAUT
- Page 68 and 69: lettersa1.5 kb hVPrIntron 112.5 kbA
- Page 70 and 71: letters© 2010 Nature America, Inc.
- Page 72 and 73: letters© 2010 Nature America, Inc.
- Page 74 and 75: l e t t e r sReal-time imaging of h
- Page 76 and 77: l e t t e r sFigure 2 Time-lapse li
- Page 78 and 79: l e t t e r s© 2010 Nature America
- Page 80 and 81: l e t t e r sRational design of cat
- Page 84 and 85: l e t t e r s© 2010 Nature America
- Page 86 and 87: sample fluorescence was measured as
- Page 88 and 89: careers and recruitmentFourth quart