Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
developmental pathway that may<br />
involve the Myb onco-protein. In<br />
support of such a speculation are our<br />
observations that murine GBX2 and<br />
FGF-2 knock-outs display epistatic<br />
hematopoietic defects and GBX2 and<br />
FGF-2 are co-expressed in<br />
hematopoietic cell lines. We are,<br />
therefore, searching for a link between<br />
GBX2, FGF-2, its receptor, and Myb.<br />
Interestingly, the same mutations in<br />
leukemogenic Myb that constitutively<br />
activate GBX2 concomitantly<br />
abrogate the collaboration between<br />
Myb and C/EBP. Accordingly, they<br />
are loss-of-function mutations for<br />
C/EBP collaboration. Since C/EBP<br />
induces cell differentiation and<br />
proliferation arrest, it appears that the<br />
oncoprotein abolishes the function of<br />
a genetic switch that controls terminal<br />
differentiation of myeloid cells.<br />
Translational regulation of<br />
transcription factors<br />
Several protein isoforms arise from<br />
both GBX2 and C/EBP mRNAs by<br />
alternative initiation of protein<br />
translation at different start codons.<br />
The isoforms give rise to DNA<br />
regulatory proteins with entirely<br />
different functions. In the case of<br />
C/EBPs, full-length proteins are transactivators<br />
while an internally initiated<br />
protein is a repressor. The C/EBP<br />
transactivator proteins mediate<br />
proliferation arrest and cellular<br />
differentiation, whereas the repressor<br />
permits proliferation. Long and short<br />
protein isoforms are also generated<br />
from the GBX2 mRNA. Unlike<br />
C/EBPs, however, long GBX2<br />
isoforms are repressors whereas the<br />
short form is an activator. The<br />
activator GBX2 supports expression<br />
of at least one cytokine that promotes<br />
precursor cell proliferation. Thus,<br />
internal start site usage will support<br />
growth because short, growthpromoting<br />
isoforms replace the long,<br />
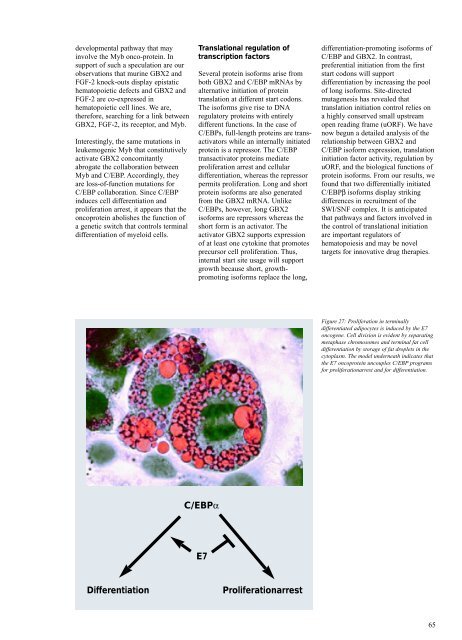
C/EBPα<br />
E7<br />
Differentiation Proliferationarrest<br />
differentiation-promoting isoforms of<br />
C/EBP and GBX2. In contrast,<br />
preferential initiation from the first<br />
start codons will support<br />
differentiation by increasing the pool<br />
of long isoforms. Site-directed<br />
mutagenesis has revealed that<br />
translation initiation control relies on<br />
a highly conserved small upstream<br />
open reading frame (uORF). We have<br />
now begun a detailed analysis of the<br />
relationship between GBX2 and<br />
C/EBP isoform expression, translation<br />
initiation factor activity, regulation by<br />
uORF, and the biological functions of<br />
protein isoforms. From our results, we<br />
found that two differentially initiated<br />
C/EBPβ isoforms display striking<br />
differences in recruitment of the<br />
SWI/SNF complex. It is anticipated<br />
that pathways and factors involved in<br />
the control of translational initiation<br />
are important regulators of<br />
hematopoiesis and may be novel<br />
targets for innovative drug therapies.<br />
Figure 27: Proliferation in terminally<br />
differentiated adipocytes is induced by the E7<br />
oncogene. Cell division is evident by separating<br />
metaphase chromosomes and terminal fat cell<br />
differentiation by storage of fat droplets in the<br />
cytoplasm. The model underneath indicates that<br />
the E7 oncoprotein uncouples C/EBP programs<br />
for proliferationarrest and for differentiation.<br />
65