You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
New modes of calcium signaling<br />
Maik Gollasch, Matthias Löhn, and<br />
Michael Fürstenau are making<br />
exciting advances in electrophysiology.<br />
During a Humboldt fellowship at the<br />
University of Vermont, Gollasch<br />
worked with Mark Nelson and studied<br />
local calcium transients termed<br />
calcium sparks. These sparks are<br />
apparently caused by opening of<br />
clustered ryanodine receptors in the<br />
sarcoplasmic reticulum. Gollasch’s<br />
team has investigated caveolae,<br />
cholesterol/sphingolipid-rich<br />
invaginations of the plasma membrane<br />
which colocalize with both the<br />
subsarcolemmal occurrence of<br />
calcium sparks and the junctional<br />
sarcoplasmic reticulum. They have<br />
found that a transient elevation in<br />
calcium at the inner mouth of a single<br />
L-type calcium channel within<br />
caveolae induces simultaneous<br />
activation and opens several<br />
ryanodine receptors to generate a local<br />
calcium spark. They are the first to<br />
show that a caveolae-calcium<br />
signaling pathway may regulate<br />
cellular functions via local ryanodine<br />
receptors in the sarcoplasmic<br />
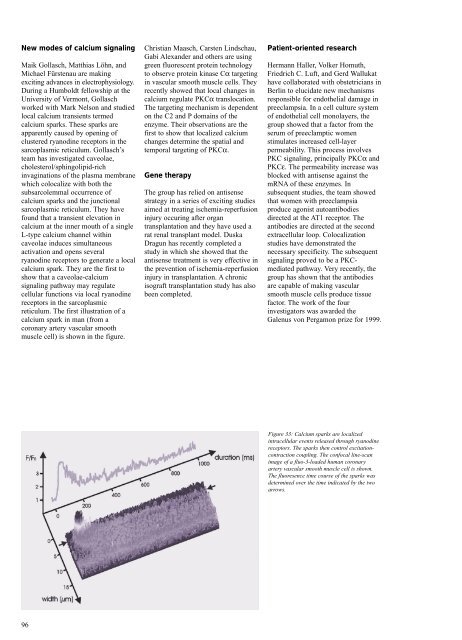
reticulum. The first illustration of a<br />
calcium spark in man (from a<br />
coronary artery vascular smooth<br />
muscle cell) is shown in the figure.<br />
96<br />
Christian Maasch, Carsten Lindschau,<br />
Gabi Alexander and others are using<br />
green fluorescent protein technology<br />
to observe protein kinase Cα targeting<br />
in vascular smooth muscle cells. They<br />
recently showed that local changes in<br />
calcium regulate PKCα translocation.<br />
The targeting mechanism is dependent<br />
on the C2 and P domains of the<br />
enzyme. Their observations are the<br />
first to show that localized calcium<br />
changes determine the spatial and<br />
temporal targeting of PKCα.<br />
Gene therapy<br />
The group has relied on antisense<br />
strategy in a series of exciting studies<br />
aimed at treating ischemia-reperfusion<br />
injury occuring after organ<br />
transplantation and they have used a<br />
rat renal transplant model. Duska<br />
Dragun has recently completed a<br />
study in which she showed that the<br />
antisense treatment is very effective in<br />
the prevention of ischemia-reperfusion<br />
injury in transplantation. A chronic<br />
isograft transplantation study has also<br />
been completed.<br />
Patient-oriented research<br />
Hermann Haller, Volker Homuth,<br />
Friedrich C. Luft, and Gerd Wallukat<br />
have collaborated with obstetricians in<br />
Berlin to elucidate new mechanisms<br />
responsible for endothelial damage in<br />
preeclampsia. In a cell culture system<br />
of endothelial cell monolayers, the<br />
group showed that a factor from the<br />
serum of preeclamptic women<br />
stimulates increased cell-layer<br />
permeability. This process involves<br />
PKC signaling, principally PKCα and<br />
PKCε. The permeability increase was<br />
blocked with antisense against the<br />
mRNA of these enzymes. In<br />
subsequent studies, the team showed<br />
that women with preeclampsia<br />
produce agonist autoantibodies<br />
directed at the AT1 receptor. The<br />
antibodies are directed at the second<br />
extracellular loop. Colocalization<br />
studies have demonstrated the<br />
necessary specificity. The subsequent<br />
signaling proved to be a PKCmediated<br />
pathway. Very recently, the<br />
group has shown that the antibodies<br />
are capable of making vascular<br />
smooth muscle cells produce tissue<br />
factor. The work of the four<br />
investigators was awarded the<br />
Galenus von Pergamon prize for 1999.<br />
Figure 33: Calcium sparks are localized<br />
intracellular events released through ryanodine<br />
receptors. The sparks then control excitationcontraction<br />
coupling. The confocal line-scan<br />
image of a fluo-3-loaded human coronary<br />
artery vascular smooth muscle cell is shown.<br />
The fluoresence time course of the sparks was<br />
determined over the time indicated by the two<br />
arrows.