California Biomedical Industry - California Healthcare Institute
California Biomedical Industry - California Healthcare Institute
California Biomedical Industry - California Healthcare Institute
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
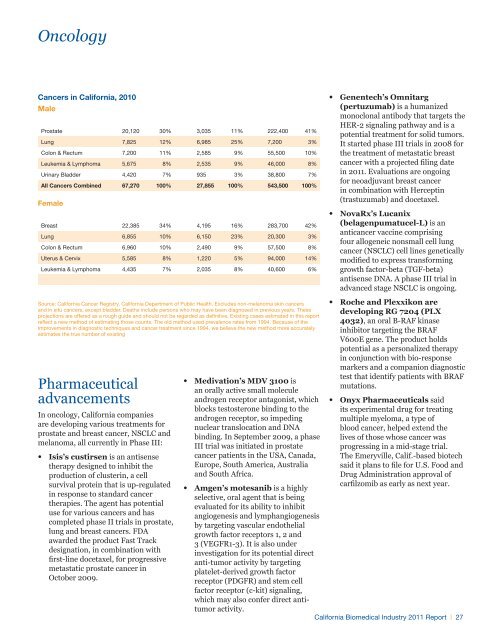
OncologyCancers in <strong>California</strong>, 2010MaleProstate 20,120 30% 3,035 11% 222,400 41%Lung 7,825 12% 6,985 25% 7,200 3%Colon & Rectum 7,200 11% 2,585 9% 55,500 10%Leukemia & Lymphoma 5,675 8% 2,535 9% 46,000 8%Urinary Bladder 4,420 7% 935 3% 38,800 7%All Cancers Combined 67,270 100% 27,855 100% 543,500 100%FemaleBreast 22,385 34% 4,195 16% 283,700 42%Lung 6,855 10% 6,150 23% 20,300 3%Colon & Rectum 6,960 10% 2,490 9% 57,500 8%Uterus & Cervix 5,585 8% 1,220 5% 94,000 14%Leukemia & Lymphoma 4,435 7% 2,035 8% 40,600 6%Source: <strong>California</strong> Cancer Registry, <strong>California</strong> Department of Public Health. Excludes non-melanoma skin cancersand in situ cancers, except bladder. Deaths include persons who may have been diagnosed in previous years. Theseprojections are offered as a rough guide and should not be regarded as definitive. Existing cases estimated in this reportreflect a new method of estimating those counts. The old method used prevalence rates from 1994. Because of theimprovements in diagnostic techniques and cancer treatment since 1994, we believe the new method more accuratelyestimates the true number of existingPharmaceuticaladvancementsIn oncology, <strong>California</strong> companiesare developing various treatments forprostate and breast cancer, NSCLC andmelanoma, all currently in Phase III:••Isis’s custirsen is an antisensetherapy designed to inhibit theproduction of clusterin, a cellsurvival protein that is up-regulatedin response to standard cancertherapies. The agent has potentialuse for various cancers and hascompleted phase II trials in prostate,lung and breast cancers. FDAawarded the product Fast Trackdesignation, in combination withfirst-line docetaxel, for progressivemetastatic prostate cancer inOctober 2009.••Medivation’s MDV 3100 isan orally active small moleculeandrogen receptor antagonist, whichblocks testosterone binding to theandrogen receptor, so impedingnuclear translocation and DNAbinding. In September 2009, a phaseIII trial was initiated in prostatecancer patients in the USA, Canada,Europe, South America, Australiaand South Africa.••Amgen’s motesanib is a highlyselective, oral agent that is beingevaluated for its ability to inhibitangiogenesis and lymphangiogenesisby targeting vascular endothelialgrowth factor receptors 1, 2 and3 (VEGFR1-3). It is also underinvestigation for its potential directanti-tumor activity by targetingplatelet-derived growth factorreceptor (PDGFR) and stem cellfactor receptor (c-kit) signaling,which may also confer direct antitumoractivity.••Genentech’s Omnitarg(pertuzumab) is a humanizedmonoclonal antibody that targets theHER-2 signaling pathway and is apotential treatment for solid tumors.It started phase III trials in 2008 forthe treatment of metastatic breastcancer with a projected filing datein 2011. Evaluations are ongoingfor neoadjuvant breast cancerin combination with Herceptin(trastuzumab) and docetaxel.••NovaRx’s Lucanix(belagenpumatucel-L) is ananticancer vaccine comprisingfour allogeneic nonsmall cell lungcancer (NSCLC) cell lines geneticallymodified to express transforminggrowth factor-beta (TGF-beta)antisense DNA. A phase III trial inadvanced stage NSCLC is ongoing.••Roche and Plexxikon aredeveloping RG 7204 (PLX4032), an oral B-RAF kinaseinhibitor targeting the BRAFV600E gene. The product holdspotential as a personalized therapyin conjunction with bio-responsemarkers and a companion diagnostictest that identify patients with BRAFmutations.• • Onyx Pharmaceuticals saidits experimental drug for treatingmultiple myeloma, a type ofblood cancer, helped extend thelives of those whose cancer wasprogressing in a mid-stage trial.The Emeryville, Calif.-based biotechsaid it plans to file for U.S. Food andDrug Administration approval ofcarfilzomib as early as next year.<strong>California</strong> <strong>Biomedical</strong> <strong>Industry</strong> 2011 Report | 27