acta 2_2015
acta 2_2015
acta 2_2015
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
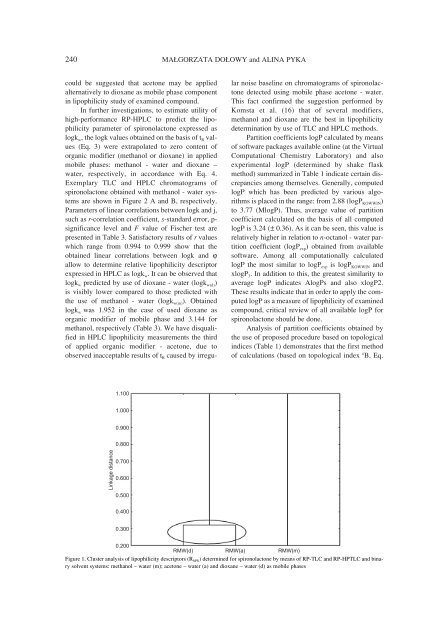
240 MA£GORZATA DO£OWY and ALINA PYKAcould be suggested that acetone may be appliedalternatively to dioxane as mobile phase componentin lipophilicity study of examined compound.In further investigations, to estimate utility ofhigh-performance RP-HPLC to predict the lipophilicityparameter of spironolactone expressed aslogk w , the logk values obtained on the basis of t R values(Eq. 3) were extrapolated to zero content oforganic modifier (methanol or dioxane) in appliedmobile phases: methanol - water and dioxane ñwater, respectively, in accordance with Eq. 4.Exemplary TLC and HPLC chromatograms ofspironolactone obtained with methanol - water systemsare shown in Figure 2 A and B, respectively.Parameters of linear correlations between logk and j,such as r-correlation coefficient, s-standard error, p-significance level and F value of Fischer test arepresented in Table 3. Satisfactory results of r valueswhich range from 0.994 to 0.999 show that theobtained linear correlations between logk and ϕallow to determine relative lipophilicity descriptorexpressed in HPLC as logk w . It can be observed thatlogk w predicted by use of dioxane - water (logk w(d) )is visibly lower compared to those predicted withthe use of methanol - water (logk w(m) ). Obtainedlogk w was 1.952 in the case of used dioxane asorganic modifier of mobile phase and 3.144 formethanol, respectively (Table 3). We have disqualifiedin HPLC lipophilicity measurements the thirdof applied organic modifier - acetone, due toobserved inacceptable results of t R caused by irregularnoise baseline on chromatograms of spironolactonedetected using mobile phase acetone - water.This fact confirmed the suggestion performed byKomsta et al. (16) that of several modifiers,methanol and dioxane are the best in lipophilicitydetermination by use of TLC and HPLC methods.Partition coefficients logP calculated by meansof software packages available online (at the VirtualComputational Chemistry Laboratory) and alsoexperimental logP (determined by shake flaskmethod) summarized in Table 1 indicate certain discrepanciesamong themselves. Generally, computedlogP which has been predicted by various algorithmsis placed in the range: from 2.88 (logP KOWWIN )to 3.77 (MlogP). Thus, average value of partitioncoefficient calculated on the basis of all computedlogP is 3.24 (± 0.36). As it can be seen, this value isrelatively higher in relation to n-octanol - water partitioncoefficient (logP exp ) obtained from availablesoftware. Among all computationally calculatedlogP the most similar to logP exp is logP KOWWIN andxlogP 3 . In addition to this, the greatest similarity toaverage logP indicates AlogPs and also xlogP2.These results indicate that in order to apply the computedlogP as a measure of lipophilicity of examinedcompound, critical review of all available logP forspironolactone should be done.Analysis of partition coefficients obtained bythe use of proposed procedure based on topologicalindices (Table 1) demonstrates that the first methodof calculations (based on topological index 0 B, Eq.Figure 1. Cluster analysis of lipophilicity descriptors (R MW ) determined for spironolactone by means of RP-TLC and RP-HPTLC and binarysolvent systems: methanol ñ water (m); acetone ñ water (a) and dioxane ñ water (d) as mobile phases