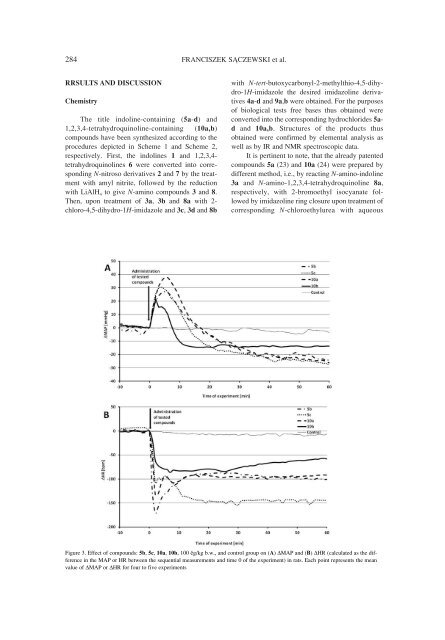
284 FRANCISZEK S•CZEWSKI et al.RRSULTS AND DISCUSSIONChemistryThe title indoline-containing (5a-d) and1,2,3,4-tetrahydroquinoline-containing (10a,b)compounds have been synthesized according to theprocedures depicted in Scheme 1 and Scheme 2,respectively. First, the indolines 1 and 1,2,3,4-tetrahydroquinolines 6 were converted into correspondingN-nitroso derivatives 2 and 7 by the treatmentwith amyl nitrite, followed by the reductionwith LiAlH 4 to give N-amino compounds 3 and 8.Then, upon treatment of 3a, 3b and 8a with 2-chloro-4,5-dihydro-1H-imidazole and 3c, 3d and 8bwith N-tert-butoxycarbonyl-2-methylthio-4,5-dihydro-1H-imidazolethe desired imidazoline derivatives4a-d and 9a,b were obtained. For the purposesof biological tests free bases thus obtained wereconverted into the corresponding hydrochlorides 5adand 10a,b. Structures of the products thusobtained were confirmed by elemental analysis aswell as by IR and NMR spectroscopic data.It is pertinent to note, that the already patentedcompounds 5a (23) and 10a (24) were prepared bydifferent method, i.e., by reacting N-amino-indoline3a and N-amino-1,2,3,4-tetrahydroquinoline 8a,respectively, with 2-bromoethyl isocyanate followedby imidazoline ring closure upon treatment ofcorresponding N-chloroethylurea with aqueousFigure 3. Effect of compounds: 5b, 5c, 10a, 10b, 100 Ïg/kg b.w., and control group on (A) ∆MAP and (B) ∆HR (calculated as the differencein the MAP or HR between the sequential measurements and time 0 of the experiment) in rats. Each point represents the meanvalue of ∆MAP or ∆HR for four to five experiments
1-[(Imidazolin-2-yl)amino]indoline and 1-[(imidazolin-2-yl)amino]1,2,3,4-tetrahydroquinoline... 285NaOH. No spectral data for both the free bases orcorresponding hydrochloride salts have previouslybeen described.Binding affinities at α 1 -, α 2 -adrenoceptors andimidazoline I 1 and I 2 receptorsRadioligand binding experiments of α 2 -adrenoceptors and imidazoline I 2 receptors wereconducted using crude P 2 rat brain membranes, andcrude P 2 rat kidney membranes were used for I 1receptors. Equilibrium dissociation constants (K i )were determined by the method of Cheng & Prusoff(35) and the resulting values are presented in Table1 as the mean ± SEM for 3 or 4 separate experiments.As shown in Table 1, the unsubstituted compound5a showed a poor affinity for both the α 1 - andα 2 -adrenoceptors with K i = 1340 nM and 3640 nM,respectively. Compound 5b with CH 3 substituent atposition 2 displayed enhanced activity at α 1 - (K i =30.2 nM) and α 2 - (K i = 6.09 nM) receptors, but stilla negligible α 1 /α 2 selectivity ratio of 4.96. The highestdifference in potencies at α 1 - and α 2 -adrenoceptorswas showed by 7-CH 3 and 4-Cl -substitutedindolines 5c and 5d (α 2 K i = 0.75 and 3.1 nM,respectively; α 1 /α 2 selectivity ratio = 36.19 and34.24, respectively). It should be pointed out thatunsubstituted 1,2,3,4-tetrahydroisoquinoline compound10a also displayed good affinity for α 2 -adrenoceptors (K i = 4.94 nM) and a moderate affinityfor α 1 -adrenoceptor (K i = 69.8 nM), while the 8-CH 3 congener 10b proved to be less potent.It is worth mentioning here the noticeableaffinities of indoline 5b at imidazoline I 1 receptors(IC 50 = 39.7 nM) and 1,2,3,4-tetrahydroquinoline10a at imidazoline I 2 receptors (K i = 14.4 nM).Effect on arterial blood pressure and heart rateIntravenous administration of compounds 5b,cand 10a,b at dose 0.1 mg/kg in thiopental-anesthetizedmale Wistar rats caused a short-lasting pressorresponse after which significant reduction of arterialblood pressure was observed (Table 2, Fig. 3A).The most pronounced changes in blood pressure wereobserved for indoline 5c and 1,2,3,4-tetrahydroquinolinederivative 10a, i.e., the compounds with relativelyhigh α 1 - and α 2 -adrenoceptor affinities (Table 1).The initially observed increase in blood pressureresulting from activation of vascular α 1 -adrenoceptors(Table 2, Fig. 3A, ∆MAP = 29 and 37 mmHg,respectively) was followed by a long-lasting hypotensiveeffect (∆MAP = -28 and -26 mmHg, respectively).Thus, in the circulatory system of Wistar rats theinvestigated compounds behaved as nonselectivestimulators of both α 1 - and α 2 -adrenoceptors, whichwas further confirmed by a pronounced bradycardiceffect elicited by these compounds (Table 3, Fig. 3B,∆HR = -154 and -133 bpm, respectively).The negative chronotropic effect observed in thepresent study deserves a special attention. It is wellknown that the human heart expresses α 1 -adrenoceptorsalbeit at much lower levels than β-adrenoceptors(37). However, the role of α 1 -receptors in cardiacphysiology is still a matter of debate, contrary to theirwell established effects in regulation of blood flow byinducing constriction of major arteries smooth muscles(38). Very recent study on papillary musclesobtained from rat heart ventricles indicated that stimulationof α 1 -adrenoceptors inhibits cardiac excitation-contractioncoupling through tyrosine phosphorylationof β 1 -adrenoceptors (39). Moreover, experimentsperformed on human cardiac myocytes indicatedexpression of α 1A - and α 1B -adrenoceptors subtypes(40) that are considered as cardioprotective proteins(41). In view of the above information, the immediatesharp fall in the heart rate observed after intravenousadministration of tested compounds (Table 3, Fig.3B) might possibly be mediated by cardiac α 1 -adrenoceptors activation.On the other hand, cardiac function is under thecontrol of the sympathetic and parasympatheticnervous systems. Whereas sympathetic stimulationleads to an increase of cardiac function, the effectsof the parasympathetic system are the opposite andvagal stimulation exerts negative inotropic, negativechronotropic and negative chromotropic effects inthe heart (42). These observations stay in agreementwith our recent experiments performed on vagotomizedrats, indicating that although cardiovasculareffects of imidazoline compunds of type B (Fig. 1, X= NH, marsanidine, 7-Me-marsanidine) are notmediated by the vagal nerves, vagotomy enhancedthe sensitivity of sympathetic pathways for testedcompounds (43). Therefore, the long-lasting heartrate decrease and hypotensive effect of tested compoundsmight be mediated through activation of thecentral α 2 -adrenoceptors and the subsequentdecrease of sympathetic activity [44, 45].CONCLUSIONIn conclusion, the present studies extend theresults previously described in patent literature,showing that imidazoline-containing indolines 5 and1,2,3,4-tetrahydroquinolines 10 administered at doseof 0.1 mg/kg i.v. elicit long-lasting hypotensive andbradycardic effects attributable to their ability to stimulatecentral α 2 -adrenoceptors, and therefore, should