Dissertations in Forestry and Natural Sciences
24lYKFN
24lYKFN
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Taneli Väisänen: Effects of Thermally Extracted Wood Distillates on<br />
the Characteristics of Wood-Plastic Composites<br />
polymer will be strong but brittle, which accounts for the high<br />
modulus <strong>and</strong> low impact resistance (Galeski 2003).<br />
Semicrystall<strong>in</strong>e polymers have both crystall<strong>in</strong>e <strong>and</strong> amorphous<br />
regions, i.e., these polymers comb<strong>in</strong>e the high strength of<br />
crystall<strong>in</strong>e polymers with the flexibility of the amorphous types<br />
(Callister 2005). In composite materials, semicrystall<strong>in</strong>e<br />
polymers are typically more efficiently re<strong>in</strong>forced by fibers than<br />
amorphous ones because the fibers act as nucleation sites for the<br />
crystallization process with the fiber becom<strong>in</strong>g surrounded by<br />
a f<strong>in</strong>ely divided microcrystall<strong>in</strong>e structure, which improves the<br />
modulus, especially the flexural modulus (Quan et al. 2005).<br />
At the moment, PEs are the most commonly used plastics<br />
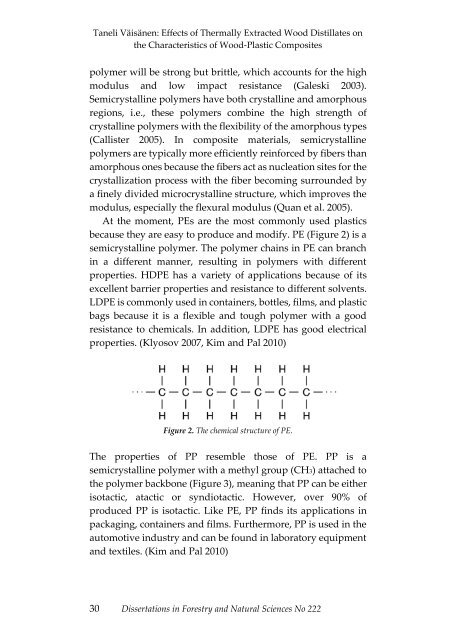
because they are easy to produce <strong>and</strong> modify. PE (Figure 2) is a<br />
semicrystall<strong>in</strong>e polymer. The polymer cha<strong>in</strong>s <strong>in</strong> PE can branch<br />
<strong>in</strong> a different manner, result<strong>in</strong>g <strong>in</strong> polymers with different<br />
properties. HDPE has a variety of applications because of its<br />
excellent barrier properties <strong>and</strong> resistance to different solvents.<br />
LDPE is commonly used <strong>in</strong> conta<strong>in</strong>ers, bottles, films, <strong>and</strong> plastic<br />
bags because it is a flexible <strong>and</strong> tough polymer with a good<br />
resistance to chemicals. In addition, LDPE has good electrical<br />
properties. (Klyosov 2007, Kim <strong>and</strong> Pal 2010)<br />
Figure 2. The chemical structure of PE.<br />
The properties of PP resemble those of PE. PP is a<br />
semicrystall<strong>in</strong>e polymer with a methyl group (CH3) attached to<br />
the polymer backbone (Figure 3), mean<strong>in</strong>g that PP can be either<br />
isotactic, atactic or syndiotactic. However, over 90% of<br />
produced PP is isotactic. Like PE, PP f<strong>in</strong>ds its applications <strong>in</strong><br />
packag<strong>in</strong>g, conta<strong>in</strong>ers <strong>and</strong> films. Furthermore, PP is used <strong>in</strong> the<br />
automotive <strong>in</strong>dustry <strong>and</strong> can be found <strong>in</strong> laboratory equipment<br />
<strong>and</strong> textiles. (Kim <strong>and</strong> Pal 2010)<br />
30 <strong>Dissertations</strong> <strong>in</strong> <strong>Forestry</strong> <strong>and</strong> <strong>Natural</strong> <strong>Sciences</strong> No 222