Staff Members of the Institute of Biochemistry, TU - Institut für ...
Staff Members of the Institute of Biochemistry, TU - Institut für ...
Staff Members of the Institute of Biochemistry, TU - Institut für ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
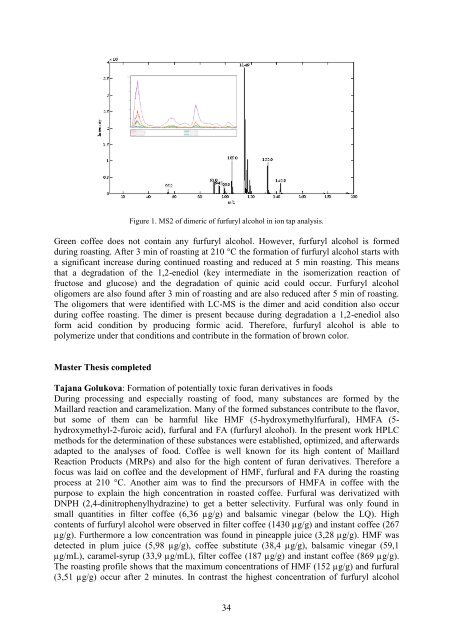
Figure 1. MS2 <strong>of</strong> dimeric <strong>of</strong> furfuryl alcohol in ion tap analysis.<br />
Green c<strong>of</strong>fee does not contain any furfuryl alcohol. However, furfuryl alcohol is formed<br />
during roasting. After 3 min <strong>of</strong> roasting at 210 °C <strong>the</strong> formation <strong>of</strong> furfuryl alcohol starts with<br />
a significant increase during continued roasting and reduced at 5 min roasting. This means<br />
that a degradation <strong>of</strong> <strong>the</strong> 1,2-enediol (key intermediate in <strong>the</strong> isomerization reaction <strong>of</strong><br />
fructose and glucose) and <strong>the</strong> degradation <strong>of</strong> quinic acid could occur. Furfuryl alcohol<br />
oligomers are also found after 3 min <strong>of</strong> roasting and are also reduced after 5 min <strong>of</strong> roasting.<br />
The oligomers that were identified with LC-MS is <strong>the</strong> dimer and acid condition also occur<br />
during c<strong>of</strong>fee roasting. The dimer is present because during degradation a 1,2-enediol also<br />
form acid condition by producing formic acid. Therefore, furfuryl alcohol is able to<br />
polymerize under that conditions and contribute in <strong>the</strong> formation <strong>of</strong> brown color.<br />
Master Thesis completed<br />
Tajana Golukova: Formation <strong>of</strong> potentially toxic furan derivatives in foods<br />
During processing and especially roasting <strong>of</strong> food, many substances are formed by <strong>the</strong><br />
Maillard reaction and caramelization. Many <strong>of</strong> <strong>the</strong> formed substances contribute to <strong>the</strong> flavor,<br />
but some <strong>of</strong> <strong>the</strong>m can be harmful like HMF (5-hydroxymethylfurfural), HMFA (5hydroxymethyl-2-furoic<br />
acid), furfural and FA (furfuryl alcohol). In <strong>the</strong> present work HPLC<br />
methods for <strong>the</strong> determination <strong>of</strong> <strong>the</strong>se substances were established, optimized, and afterwards<br />
adapted to <strong>the</strong> analyses <strong>of</strong> food. C<strong>of</strong>fee is well known for its high content <strong>of</strong> Maillard<br />
Reaction Products (MRPs) and also for <strong>the</strong> high content <strong>of</strong> furan derivatives. Therefore a<br />
focus was laid on c<strong>of</strong>fee and <strong>the</strong> development <strong>of</strong> HMF, furfural and FA during <strong>the</strong> roasting<br />
process at 210 °C. Ano<strong>the</strong>r aim was to find <strong>the</strong> precursors <strong>of</strong> HMFA in c<strong>of</strong>fee with <strong>the</strong><br />
purpose to explain <strong>the</strong> high concentration in roasted c<strong>of</strong>fee. Furfural was derivatized with<br />
DNPH (2,4-dinitrophenylhydrazine) to get a better selectivity. Furfural was only found in<br />
small quantities in filter c<strong>of</strong>fee (6,36 µg/g) and balsamic vinegar (below <strong>the</strong> LQ). High<br />
contents <strong>of</strong> furfuryl alcohol were observed in filter c<strong>of</strong>fee (1430 µg/g) and instant c<strong>of</strong>fee (267<br />
µg/g). Fur<strong>the</strong>rmore a low concentration was found in pineapple juice (3,28 µg/g). HMF was<br />
detected in plum juice (5,98 µg/g), c<strong>of</strong>fee substitute (38,4 µg/g), balsamic vinegar (59,1<br />
µg/mL), caramel-syrup (33,9 µg/mL), filter c<strong>of</strong>fee (187 µg/g) and instant c<strong>of</strong>fee (869 µg/g).<br />
The roasting pr<strong>of</strong>ile shows that <strong>the</strong> maximum concentrations <strong>of</strong> HMF (152 µg/g) and furfural<br />
(3,51 µg/g) occur after 2 minutes. In contrast <strong>the</strong> highest concentration <strong>of</strong> furfuryl alcohol<br />
34