Biennial Report 2016/2017
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Selected Results<br />
Tailoring Membrane Surface Charges: How Electrostatic Interactions<br />
Dominate Membrane Fouling<br />
D. Breite, M. Went, A. Prager, I. Thomas, A. Schulze<br />
A major problem that affects membranes in an<br />
aqueous environment is fouling. Material from the<br />
surrounding media is adsorbed to the membrane.<br />
The porous structure of the membranes is<br />
therefore blocked and the permeability and the<br />
overall performance of the membrane decrease.<br />
According to literature the cause of these<br />
adsorption processes are hydrophobic and<br />
electrostatic interactions.<br />
To further investigate the fouling caused by<br />
electrostatic interactions a new fouling test system<br />
was developed [1-3]. Anionic, neutral, and<br />
cationic polystyrene particles were synthesized<br />
and utilized for the investigation of fouling of<br />
polyvinylidene fluoride and polyethersulfone<br />
membranes. To identify the dominant interaction<br />
during fouling, the membranes were also modified<br />
using electron beam technology to gain anionic,<br />
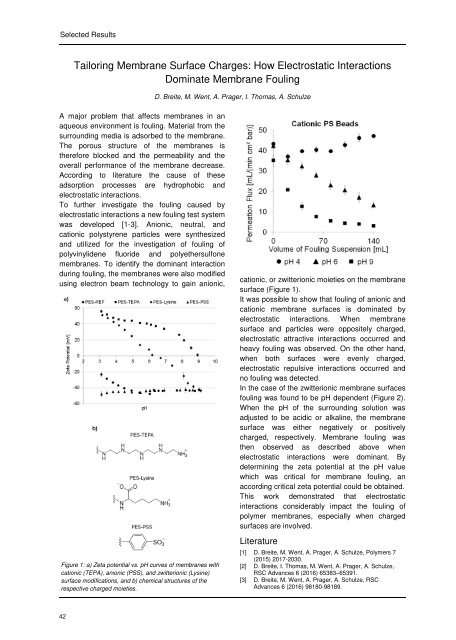
Figure 1: a) Zeta potential vs. pH curves of membranes with<br />
cationic (TEPA), anionic (PSS), and zwitterionic (Lysine)<br />
surface modifications, and b) chemical structures of the<br />
respective charged moieties.<br />
cationic, or zwitterionic moieties on the membrane<br />
surface (Figure 1).<br />
It was possible to show that fouling of anionic and<br />
cationic membrane surfaces is dominated by<br />
electrostatic interactions. When membrane<br />
surface and particles were oppositely charged,<br />
electrostatic attractive interactions occurred and<br />
heavy fouling was observed. On the other hand,<br />
when both surfaces were evenly charged,<br />
electrostatic repulsive interactions occurred and<br />
no fouling was detected.<br />
In the case of the zwitterionic membrane surfaces<br />
fouling was found to be pH dependent (Figure 2).<br />
When the pH of the surrounding solution was<br />
adjusted to be acidic or alkaline, the membrane<br />
surface was either negatively or positively<br />
charged, respectively. Membrane fouling was<br />
then observed as described above when<br />
electrostatic interactions were dominant. By<br />
determining the zeta potential at the pH value<br />
which was critical for membrane fouling, an<br />
according critical zeta potential could be obtained.<br />
This work demonstrated that electrostatic<br />
interactions considerably impact the fouling of<br />
polymer membranes, especially when charged<br />
surfaces are involved.<br />
Literature<br />
[1] D. Breite, M. Went, A. Prager, A. Schulze, Polymers 7<br />
(2015) <strong>2017</strong>-2030.<br />
[2] D. Breite, I. Thomas, M. Went, A. Prager, A. Schulze,<br />
RSC Advances 6 (<strong>2016</strong>) 65383–65391.<br />
[3] D. Breite, M. Went, A. Prager, A. Schulze, RSC<br />
Advances 6 (<strong>2016</strong>) 98180-98189.<br />
42