Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Volume 29 No. 2<br />
<strong>Summer</strong> <strong>2019</strong><br />
<strong>Gastroenterology</strong> <strong>Today</strong><br />
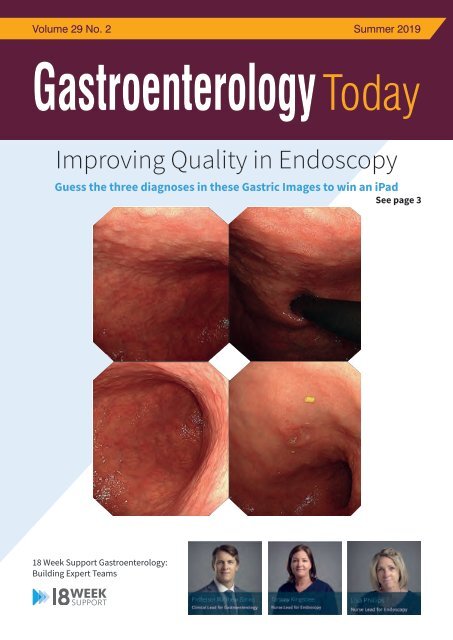
Improving Quality in Endoscopy<br />
Guess the three diagnoses in these Gastric Images to win an iPad<br />
See page 3<br />
What approach has 18 Week Support<br />
taken with regards to building an<br />
expert insourcing team?<br />
Matthew’s Perspective:<br />
Dr Matthew Banks is the Clinical Director for 18 Week Support <strong>Gastroenterology</strong>. He believes it starts with recruiting the<br />
best clinicians. ‘At 18 Week Support we set the bar very high. We only recruit clinicians whose JAG performance data is well<br />
above the national standards. In addition, we monitor each clinician’s KPIs while they work with 18 WS. While the JAG data<br />
is an excellent quality indicator, we now want to go a step beyond that and monitor the Non-Technical skills (NTS) of each<br />
clinician as well. We now know that NTS plays an important role in safe and effective team performance. Therefore, in our<br />
quest to develop excellent teams who deliver a world-class service, we must focus on NTS’.<br />
Tammy and Lisa’s Perspective:<br />
Tammy Kingstree is Lead Nurse for Endoscopy.<br />
‘It is extremely important that there are good working relationships within the team. This starts with strong leadership from<br />
our senior nurse coordinators who are trained to manage the patient pathway, manage a team of staff they may not know<br />
and to deal effectively with any issues which may arise on the day’.<br />
Lisa Phillips is Lead Nurse for Endoscopy.<br />
‘The team objectives are clear. Excellent patient experience and good patient outcomes. Because the objectives are clear,<br />
team cohesion and focus are exceptionally good. It therefore shouldn’t matter that we are in an unfamiliar endoscopy unit,<br />
the service should be seamless. If it isn’t, we do not stop until we get it right.<br />
18 Week Support <strong>Gastroenterology</strong>:<br />
Building Expert Teams<br />
If you have an excellent NHS record and want to help clear NHS waiting list backlogs, reduce RTT waiting times and provide<br />
high-quality patient care, get in touch by calling on 020 3892 6162 or email Gastro.Recruitment@18weeksupport.com
One 5-ASA<br />
stands out<br />
from the crowd;<br />
so your UC patients<br />
don’t have to.<br />
Unique among 5-ASAs, only PENTASA has ethyl cellulose coated microgranules that release<br />
mesalazine independent of pH. 1-10 Alongside this, PENTASA:<br />
• Is effective in 2 weeks 11,12 and remission is maintained for 12 months 13,14<br />
• Is effective throughout the entire colon including left-sided disease 1,13-15<br />
• Offers a broad range of formulations allowing high dose * ,<br />
once-daily dosing: 4 g – active, 2 g – remission 1,16-21<br />
Giving your mild to moderate UC patients the confidence to enjoy life.<br />
*1 g tablets, 1 g, 2 g and 4 g sachets<br />
Prescribing Information: Pentasa ® all formulations. Please consult the full<br />
Summary of Product Characteristics before prescribing. Name of Product(s):<br />
Pentasa ® Sachet prolonged release granules 1g, 2g and 4g; Pentasa ® Slow Release<br />
Tablets 500mg and 1g; Pentasa® Mesalazine Enema 1g; Pentasa ® Suppositories 1g.<br />
Composition: Sachets: contain 1g, 2g or 4g mesalazine. Tablets: contain 500mg<br />
or 1g mesalazine. Enema: contains 1g mesalazine in 100ml of aqueous suspension.<br />
Suppositories: contain 1g mesalazine. Indication: Sachets and Tablets: Mild to<br />
moderate ulcerative colitis. Enema: ulcerative colitis affecting the distal colon and<br />
rectum. Suppositories: ulcerative proctitis. Dosage: Sachets and Tablets: Adults:<br />
Active disease: up to 4g once daily or in 2–4 divided doses. Maintenance treatment:<br />
2g once daily. Sachets and 500mg tablet: Children over 6 years old: Active disease:<br />
individual dosing, starting with 30-50 mg/kg/day in divided doses (total dose should<br />
not exceed 4g/day). Maintenance treatment: individual dosing, starting with 15-30<br />
mg/kg/day in divided doses (total dose should not exceed 2g/day). Enema: Adults:<br />
one enema at bedtime. Suppositories: Adults: 1 suppository daily.Contraindications:<br />
patients with known hypersensitivity to salicylates or any of the excipients and patients<br />
with severe liver and/or renal impairment. Special Warnings and Precautions:<br />
Blood tests (differential blood count: liver function parameters such as ALT or AST;<br />
serum creatinine) and urinary status should be determined prior to and during<br />
treatment, at the discretion of the treating physician. Caution is recommended in<br />
patients with impaired hepatic function. PENTASA should not be used in patients<br />
with impaired renal function. Mesalazine- induced renal toxicity should be considered,<br />
if renal function deteriorates during treatment. Patients with pulmonary disease, in<br />
particular asthma, should be very carefully monitored during a course of treatment<br />
with PENTASA. Patients with a history of adverse drug reactions to preparations<br />
containing sulphasalazine (risk of allergy to salicylates), should be kept under close<br />
medical surveillance on commencement of a course of treatment with PENTASA.<br />
Should PENTASA cause acute intolerance reactions such as abdominal cramps,<br />
acute abdominal pain, fever, severe headache and rash, the treatment should be<br />
discontinued immediately. Mesalazine-induced cardiac hypersensitivity reactions<br />
(myo- and pericarditis) have been reported rarely. Treatment should be discontinued<br />
on suspicion or evidence of these reactions. In patients who are concomitantly treated<br />
with azathioprine, or 6-mercaptopurine, or thioguanine, a possible increase in the<br />
myelosuppressive effects of azathioprine, or 6-mercaptopurine, or thioguanine<br />
should be taken into account. There may be a decrease in the anticoagulant effect<br />
of warfarin. Do not use during pregnancy and lactation except when the potential<br />
benefits outweigh the possible risk. Sachets: Caution is recommended in patients with<br />
active peptic ulcer disease. The concurrent use of other known nephrotoxic agents,<br />
such as NSAID’s and azathioprine, may increase the risk of other renal reactions.<br />
Enema and Suppositories: If a patient develops dehydration while on treatment with<br />
mesalazine, normal electrolyte levels and fluid balance should be restored as soon as<br />
possible. Side effects: For the full list of side effects please consult the Summaries of<br />
Product Characteristics. PENTASA 1g 2g 4g sachets: Common: Headache, Diarrhoea,<br />
Abdominal pain, Nausea, Vomiting, Flatulence. Rare: Acute pancreatitis. Very rare:<br />
Benign intracranial hypertension, Pericardial effusion, Quincke’s oedema, Dermatitis<br />
allergic, Hypersensitivity reaction including anaphylactic reaction, Drug Reaction<br />
with Eosinophilia and Systemic Symptoms (DRESS). Pentasa all formulations: Rare:<br />
Dizziness, Myocarditis, Pericarditis, Photosensitivity. Very rare: Altered blood counts,<br />
Hypersensitivity reaction such as Allergic exanthema, Drug fever, Lupus erythematosus<br />
syndrome, Pancolitis, Peripheral neuropathy, Allergic and Fibrotic lung reactions,<br />
Changes in liver function parameters, Hepatitis and Cholestatic hepatitis, (Reversible)<br />
Alopecia, Renal function impairment interstitial, nephritis (incl. acute and chronic) Renal<br />
insufficiency, reversible Oligospermia. PENTASA 1g 2g 4g sachets, 1g enema and 1g<br />
suppository: Common: Rash. Rare: Increased amylase. Very rare: Cirrhosis, Hepatic<br />
failure, Erythema multiforme Stevens Johnson Syndrome (SJS), Nephrotic syndrome,<br />
Urine discolouration. PENTASA 500mg and 1g tablets, 1g enema and 1g suppository:<br />
Rare: Abdominal pain, Diarrhoea, Flatulence, Nausea, Vomiting, Headache. Very Rare:<br />
Acute Pancreatitis. Nature and Contents of Container: Sachets: Cartons contain 50 x<br />
1g sachets, 60 x 2g sachets or 30 x 4g sachets. Tablets: Cartons contain 100 x 500mg and<br />
60 x 1g tablets in blister strips. Enema: Cartons contain 7 x 100ml enemas. Suppositories:<br />
Cartons contain 28 x 1g suppositories in blister strips.Marketing Authorisation<br />
Number: Sachet 1g: 03194/0075. Sachet 2g: 03194/0102. Sachet 4g: PL 03194/0117.<br />
Tablets 500mg: 03194/0044. Tablets 1g: 3194/0108. Enema: 03194/0027. Suppositories:<br />
03194/0045. Marketing Authorisation Holder: Ferring Pharmaceuticals Ltd., Drayton<br />
Hall, Church Road, West Drayton, UB7 7PS, United Kingdom. Legal Category: POM.<br />
Basic NHS Price: £30.74 for 50 x 1g sachets. £73.78 for 60 x 2g sachets. £73.78 for<br />
30 x 4g sachets. £30.74 for 100 x 500mg Tablets. £36.89 for 60 x 1g Tablets. £17.73<br />
for 7 x enemas. £40.01 for 28 x 1g suppositories. Date of Preparation of Prescribing<br />
Information: January <strong>2019</strong>. Pentasa ® is a registered trademark. PA/035/<strong>2019</strong>/UK.<br />
Adverse events should be reported. Reporting forms<br />
and information can be found at www.mhra.gov.uk/<br />
yellowcard. Adverse events should also be reported<br />
to Ferring Pharmaceuticals Ltd. Tel: 0800 111 4126.<br />
Email: medical@ferring.com<br />
References: 1. Pentasa Slow Release Tablets 500 mg. SmPC.<br />
2. Sulfasalazine 250 mg/5 ml Oral Suspension. SmPC. 3. Octasa 400<br />
mg MR Tablets. SmPC. 4. Asacol 400 mg MR Tablets. SmPC. 5. Mezavant<br />
XL 1200 mg, Gastro-resistant, Prolonged Release Tablets. SmPC.<br />
6. Salofalk 500 mg Gastro-resistant Prolonged Release Granules. SmPC.<br />
7. Colazide 750 mg Capsules. SmPC. 8. Olsalazine Sodium 250 mg Capsules.<br />
SmPC. 9. Salazopyrin En-Tabs. SmPC. 10. Salazopyrin Tablets SmPC.<br />
11. Probert C.S.J, et al. J Crohn’s Colitis. 2014;8:200–7. 12. Marteau P, et al. Gut.<br />
2005;54(7):960–5. 13. Dignass AU, et al. Clin Gastroenterol Hepatol. 2009;7(7):762–<br />
9. 14. Bokemeyer B, et al. J Crohn’s Colitis. 2012;6:476-82. 15. Flourie B,<br />
et al. Aliment Pharmacol Ther. 2013;37(8):767–75. 16. Pentasa Slow Release Tablets 1 g.<br />
SmPC. 17. Pentasa Sachet 1 g. SmPC. 18. Pentasa Sachet 2 g. SmPC. 19. Pentasa<br />
Sachet 4 g. SmPC. 20. Pentasa Enema 1 g. SmPC. 21. Pentasa Suppositories 1 g. SmPC.<br />
Date of preparation: May <strong>2019</strong>. Job code: PA/323/<strong>2019</strong>/UKa<br />
MAKING A<br />
Positive UC action. Positive UC outcomes.<br />
DIFFERENCE<br />
Positive UC a
CONTENTSMatthew’s Perspective:<br />
5 EDITORS COMMENT<br />
6 CASE REPORT Primary Aorto-enteric Fistula<br />
14 FEATURE Showing the true value of probiotics<br />
17 NEWS<br />
26 COMPANY NEWS<br />
What approach has 18 Week Support<br />
CONTENTS<br />
taken with regards to building an<br />
expert insourcing team?<br />
<strong>Gastroenterology</strong> <strong>Today</strong><br />
Dr Matthew Banks is the Clinical Director for 18 Week Support <strong>Gastroenterology</strong>. He believes it starts with recruiting the<br />
best clinicians. ‘At 18 Week Support we set the bar very high. We only recruit clinicians whose JAG performance data is well<br />
above the national standards. In addition, we monitor each clinician’s KPIs while they work with 18 WS. While the JAG data<br />
is an excellent quality indicator, we now want to go a step beyond that and monitor the Non-Technical skills (NTS) of each<br />
clinician as well. We now know that NTS plays an important role in safe and This effective issue team performance. edited by: Therefore, in our<br />
quest to develop excellent teams who deliver a world-class service, we must focus on NTS’.<br />
Dr Ben Shandro<br />
Tammy and Lisa’s Perspective:<br />
c/o Media Publishing Company<br />
Tammy Kingstree is Lead Nurse for Endoscopy.<br />
Media House<br />
‘It is extremely important that there are good working relationships within the team. This starts with strong leadership from<br />
48 High Street<br />
our senior nurse coordinators who are trained to manage the patient pathway, manage a team of staff they may not know<br />
SWANLEY, Kent BR8 8BQ<br />
and to deal effectively with any issues which may arise on the day’.<br />
Lisa Phillips is Lead Nurse for Endoscopy.<br />
ADVERTISING & CIRCULATION:<br />
‘The team objectives are clear. Excellent patient experience and good patient outcomes. Because the objectives are clear,<br />
Media Publishing Company<br />
team cohesion and focus are exceptionally good. It therefore shouldn’t matter that we are in an unfamiliar endoscopy unit,<br />
the service should be seamless. If it isn’t, we do not stop until we get it right. Media House, 48 High Street<br />
SWANLEY, Kent, BR8 8BQ<br />
If you have an excellent NHS record and want to help clear NHS waiting list Tel: backlogs, 01322 reduce 660434 RTT waiting Fax: times 01322 and provide 666539<br />
high-quality patient care, get in touch by calling on 020 3892 6162 or email Gastro.Recruitment@18weeksupport.com<br />
E: info@mediapublishingcompany.com<br />
www.MediaPublishingCompany.com<br />
COVER STORY<br />
What are the three diagnoses evident in these<br />
gastric images?<br />
Submit your answers to jawuku@18weeksupport.com to enter our prize<br />
draw to win an iPad! The Competition will end on the 21st of June.<br />
The winner will be announced on the 18 Week Support website.<br />
What approach has 18 Week Support taken with regards to building an<br />
expert insourcing team?<br />
Matthew’s Perspective:<br />
Dr Matthew Banks is the Clinical Director for 18 Week Support <strong>Gastroenterology</strong>.<br />
He believes it starts with recruiting the best clinicians. ‘At 18 Week Support we<br />
set the bar very high. We only recruit clinicians whose JAG performance data<br />
is well above the national standards. In addition, we monitor each clinician’s<br />
KPIs while they work with 18 WS. While the JAG data is an excellent quality<br />
indicator, we now want to go a step beyond that and monitor the Non-Technical<br />
skills (NTS) of each clinician as well. We now know that NTS plays an important<br />
role in safe and effective team performance. Therefore, in our quest to develop<br />
excellent teams who deliver a world-class service, we must focus on NTS’.<br />
Tammy and Lisa’s Perspective:<br />
Tammy Kingstree is Lead Nurse for Endoscopy.<br />
‘It is extremely important that there are good working relationships within the team.<br />
This starts with strong leadership from our senior nurse coordinators who are trained<br />
to manage the patient pathway, manage a team of staff they may not know and to<br />
deal effectively with any issues which may arise on the day’.<br />
Lisa Phillips is Lead Nurse for Endoscopy.<br />
‘The team objectives are clear. Excellent patient experience and good patient outcomes.<br />
Because the objectives are clear, team cohesion and focus are exceptionally good.<br />
It therefore shouldn’t matter that we are in an unfamiliar endoscopy unit, the service<br />
should be seamless. If it isn’t, we do not stop until we get it right.<br />
For more information please contact: Ian Yuill, Director of Business Development<br />
& Recruitment on 0203 869 8792 or visit www.18weeksupport.com<br />
PUBLISHING DATES:<br />
February, June and October.<br />
COPYRIGHT:<br />
Media Publishing Company<br />
Media House<br />
48 High Street<br />
SWANLEY, Kent, BR8 8BQ<br />
PUBLISHERS STATEMENT:<br />
The views and opinions expressed in<br />
this issue are not necessarily those of<br />
the Publisher, the Editors or Media<br />
Publishing Company.<br />
Next Issue Autumn <strong>2019</strong><br />
Subscription Information – <strong>Summer</strong> <strong>2019</strong><br />
<strong>Gastroenterology</strong> <strong>Today</strong> is a tri-annual<br />
publication currently sent free of charge to<br />
all senior qualified Gastroenterologists in<br />
the United Kingdom. It is also available<br />
by subscription to other interested individuals<br />
and institutions.<br />
UK:<br />
Other medical staff - £18.00 inc. postage<br />
Non-medical Individuals - £24.00 inc. postage<br />
Institutions<br />
Libraries<br />
Commercial Organisations - £48.00 inc. postage<br />
Rest of the World:<br />
Individuals - £48.00 inc. postage<br />
Institutions<br />
Libraries<br />
Commercial Organisations - £72.00 inc. postage<br />
We are also able to process your<br />
subscriptions via most major credit<br />
cards. Please ask for details.<br />
Cheques should be made<br />
payable to MEDIA PUBLISHING.<br />
Designed in the UK by me&you creative<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
3
Getting on with<br />
their lives<br />
By getting on with<br />
their steroid<br />
For autoimmune hepatitis<br />
The only budesonide with three indications<br />
For induction of remission of mild to moderate active<br />
ileo-caecal Crohn’s disease<br />
For induction of remission of active collagenous colitis<br />
Budesonide, the Dr Falk way<br />
Efficacy localised at the site of the diseases 1-4<br />
Limiting the risk of systemic side effects 2-4<br />
Prescribing Information (Please refer to full SPC before prescribing)<br />
Presentation: Budenofalk ® gastro-resistant granules, each sachet<br />
contains 9mg budesonide, Budenofalk ® gastro-resistant capsules, each<br />
containing 3mg budesonide. Indications: Induction of remission of<br />
mild to moderate active Crohn’s disease affecting the ileum and/or the<br />
ascending colon. Induction of remission of active collagenous colitis.<br />
Autoimmune hepatitis (capsules only). Dosage: Adults: For Crohn’s<br />
disease and collagenous colitis: one sachet or three capsules daily with<br />
liquid half an hour before food, without chewing or crushing, or one<br />
capsule three times daily. Limit treatment to 8 weeks, then withdraw<br />
gradually. For autoimmune hepatitis: one capsule three times daily.<br />
Possibly combine with azathioprine. Maintenance of remission: one<br />
capsule twice daily. Revert to 3 capsules daily if transaminases ALAT and/<br />
or ASAT elevate again. Treat until remission is achieved or 24 months.<br />
Children: Not recommended; safety and efficacy not established. Contraindications:<br />
hypersensitivity to any constituent. Hepatic cirrhosis.<br />
Warnings/Precautions: Change from other steroids may result in<br />
symptoms due to reduced systemic steroids. Use with caution in patients<br />
with tuberculosis, hypertension, diabetes mellitus, osteoporosis, peptic<br />
ulcer, glaucoma, cataracts or family history of glaucoma or diabetes or any<br />
condition in which glucocorticosteroids may have undesirable effects.<br />
Not appropriate for upper GI Crohn’s or extraintestinal symptoms.<br />
Long term, high dose use may result in glucocorticosteroid systemic<br />
effects. Infection: suppression of the inflammatory response and<br />
immune function increases susceptibility to infections and their severity.<br />
Clinical presentation of infections may be atypical and presentation of<br />
serious infections may be masked. Chickenpox and herpes zoster are<br />
of particular concern. Passive immunisation needed within 10 days in<br />
exposed non-immune patients taking systemic glucocorticosteroids.<br />
Urgent specialist care required on confirmed chickenpox. Give normal<br />
immunoglobulin immediately after measles exposure. Do not give<br />
live vaccines to those with chronic glucocorticosteroid use. Antibody<br />
response to other vaccines may be diminished. With severe liver<br />
function disorders: increased systemic bioavailability. Central serous<br />
chorioretinopathy or other causes may result in blurred vision/visual<br />
disturbances. Consider referral to ophthalmologist. Suppression of<br />
the HPA axis and reduced stress response: supplementary systemic<br />
glucocorticoid treatment may be needed. Avoid concomitant treatment<br />
with CYP3A4 inhibitors. Do not use in patients with galactose or fructose<br />
intolerance, glucose – galactose malabsorption, sucrase – isomaltase<br />
insufficiency or Lapp lactase deficiency or congenital lactase deficiency.<br />
In autoimmune hepatitis evaluate transaminase levels every 2 weeks<br />
for the first month and then every 3 months. Interactions: Co-treatment<br />
with CYP3A inhibitors including cobicistat containing products may<br />
increase side effects and should be avoided where possible. Beware<br />
concomitant administration of cardiac glycosides and saluretics.<br />
CYP3A4 inhibitors: avoid concomitant administration. CYP3A4<br />
inducers: may reduce systemic and local exposure, necessitating dose<br />
adjustment of budesonide. CYP3A4 substrates: may compete with<br />
budesonide increasing plasma concentrations depending on relative<br />
affinities. Small, non-significant effect of cimetidine on budesonide<br />
kinetic effects. Oestrogens/oral contraceptives may elevate plasma<br />
concentrations and enhance corticosteroid effects. Steroid-binding<br />
compounds and antacids may reduce budesonide efficacy; administer<br />
at least 2 hours apart. Because adrenal function may be supressed,<br />
an ACTH stimulation test for diagnosing pituitary insufficiency might<br />
show false results (low values). Use in pregnancy and lactation: Avoid<br />
use in pregnancy unless essential. Do not breastfeed during Budenofalk<br />
treatment. Undesirable effects: Cushing’s syndrome, growth retardation<br />
in children, glaucoma, cataracts, blurred vision, dyspepsia, abdominal<br />
pain, constipation, gastric or duodenal ulcers, pancreatitis, increase in<br />
risk of infections, muscle and joint pain and weakness and twitching,<br />
osteoporosis, osteonecrosis, headache, pseudotumor cerebri (including<br />
papilloedema) in adolescents, depression, irritability and euphoria,<br />
www.drfalk.co.uk<br />
Dr Falk Pharma UK Ltd, Unit K, Bourne End Business Park, Cores End Rd, Bourne End, SL8 5AS. Registered in England No: 2307698<br />
psychomotor hyperactivity, anxiety, aggression, allergic exanthema,<br />
petechiae, ecchymosis, contact dermatitis, delayed wound healing,<br />
increased risk of thrombosis, vasculitis (after withdrawal from longterm<br />
treatment), fatigue, malaise. Side effects characteristic of systemic<br />
glucocorticosteroid therapy may occur. Exacerbation or reappearance of<br />
extraintestinal manifestations when switching from systemically acting<br />
glucocorticosteroids may occur. Frequency is likely to be lower than<br />
with equivalent dosage of prednisolone. Legal category: POM. Costs:<br />
UK NHS: 60 sachets £135.00; 100 capsules £75.05. Ireland (PtW):<br />
60 sachets: €149.49; 100 capsules: €78.96. Licence holder: Dr Falk<br />
Pharma GmbH, Leinenweberstr.5, D-79108 Freiburg, Germany. Licence<br />
numbers: (granules) PL08637/0020 (UK) PA573/2/3 (IE) (capsules)<br />
PL08637/0002 (UK) PA573/2/1 (IE). Prepared: February <strong>2019</strong>.<br />
Further information available on request.<br />
Adverse events should be reported. Reporting forms and information<br />
can be found at www.mhra.gov.uk/yellowcard or search for MHRA<br />
Yellow Card in the Google Play, Apple App Store (UK residents) or<br />
at email: medsafety@hpra.ie or at http://www.hpra.ie/homepage/<br />
about-us/report-an-issue/human-adverse-reaction-form (residents<br />
in Ireland). Adverse events should also be reported to Dr Falk<br />
Pharma UK Ltd.<br />
References:<br />
1. Bar-Meir S et al. Gastroenterol 1998; 115(4): 835-40. 2. De Cassan C<br />
et al. Dig Des 2012; 30(4): 368-75. 3. Czaja AJ. Dig Dis Sci 2012; 57(8):<br />
1996-2010. 4. Miehlke S et al. Gastroenterol 2002; 123(4): 978-84.<br />
DrF19/003<br />
Date of preparation: March <strong>2019</strong>
EDITORS COMMENT<br />
EDITORS COMMENT<br />
Seek and ye shall find<br />
“As with<br />
all rare<br />
diagnoses,<br />
the critical<br />
first step<br />
is to<br />
consider<br />
it.”<br />
The case-report by Arakkal et al. included in this edition of <strong>Gastroenterology</strong> <strong>Today</strong> presents<br />
a rare cause of a common presentation, and highlights the importance of including these in<br />
the differential diagnosis.<br />
Arakkal et al. describe a case of acute upper GI bleeding secondary to primary aortoenteric<br />
fistula. The diagnosis of primary aorto-enteric fistula requires cross-sectional<br />
imaging, which does not form part of the GI bleeding pathway in most international<br />
guidelines. These patients could exsanguinate whilst awaiting their colonoscopy or video<br />
capsule endoscopy, the two investigations usually advocated after negative upper GI<br />
endoscopy. If this rare diagnosis had not been considered, and the CT scan requested,<br />
then the diagnosis could not have been made.<br />
Early identification of patients with aorto-enteric fistula is paramount, as it is common<br />
to present with a smaller herald bleed prior to catastrophic GI haemorrhage. Presenting<br />
symptoms are non-specific. Routine physical examination is only moderately sensitive for<br />
detecting abdominal aortic aneurysm (AAA), even when the physical examination is directed<br />
specifically to the detection of AAA.<br />
In contrast, ultrasound and CT imaging are highly sensitive. Could there be a role for<br />
focussed ultrasound scanning in the emergency department for at-risk patients who present<br />
with GI bleeding, such as those aged over 60 years?<br />
As with all rare diagnoses, the critical first step is to consider it.<br />
Dr Ben Shandro<br />
Research Fellow in <strong>Gastroenterology</strong><br />
St George’s Hospital<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
5
CASE REPORT<br />
PRIMARY AORTO-ENTERIC FISTULA -<br />
A CASE REPORT<br />
1. Dr Mohammed Shaheer Pandara Arakkal, Senior Clinical Fellow , <strong>Gastroenterology</strong>, Morriston hospital, Swansea Wales, UK<br />
2. Dr.Umakant Dave, Consultant Gastroenterologist, , Morriston Hospital, Swansea Wales, UK<br />
3. Victoria Trainer, Consultant Radiologist, Singleton Hospital, Swansea Wales, UK<br />
Abstract<br />
Aorto-enteric fistula is a life threatening condition and is defined as<br />
an abnormal communication between the abdominal aorta and<br />
gastrointestinal tract. It is most often secondary to either erosion of an<br />
aortic prosthetic graft or other vascular procedure on the aorta, known as<br />
secondary aorto-enteric fistulae (SAEF). Primary aorto-enteric fistula (PAEF)<br />
occurs as a result of compression of an abdominal aortic aneurysm (AAA)<br />
against the surrounding gastrointestinal structures causing spontaneous<br />
communication between them. We describe a case of PAEF in an 85 year<br />
old woman who was known to have AAA who presented with acute upper<br />
gastrointestinal haemorrhage to the emergency department. This report<br />
highlights the importance of high index of suspicion in diagnosing aortoenteric<br />
fistula, especially if the patient is known to have AAA.<br />
Introduction<br />
Primary aorto-enteric fistula (PAEF) is an abnormal communication<br />
between abdominal aorta and gastrointestinal (GI) tract in the absence of<br />
any vascular intervention on aorta. Secondary aorto-enteric fistula occurs<br />
as a complication of endovascular procedures with prosthesis or stent<br />
graft or a surgical procedure usually for an AAA repair. Primary aortoenteric<br />
fistula (PAEF) is a rare but serious differential diagnosis that must<br />
be considered in any patients who presents with upper gastro intestinal<br />
(GI) haemorrhage, especially if the patient is known to have an abdominal<br />
aortic aneurysm (AAA). Although aorto-enteric fistula (AEF) has several<br />
aetiologies, the most common cause of PAEF is AAA. As PAEF is quite<br />
a challenging diagnosis for the treating physician, early detection and<br />
treatment is vital for the patient survival. If left untreated mortality is 100%.<br />
This is a case report of a patient with known AAA who was found to have<br />
Aorto- duodenal fistula while investigating for Upper GI haemorrhage.<br />
Case Report<br />
An 85 year old lady presented to the Emergency department (ED) in<br />
October 2018 with two episodes of haematemesis and one episode<br />
melaena. She had a further episode of melaena in the ED. She had a<br />
background history of coronary artery bypass graft (CABG) in 1993, chronic<br />
obstructive pulmonary disease (COPD), osteoporosis, varicose eczema<br />
and high cholesterol. She was an ex-heavy smoker with a 50 pack-year<br />
smoking history and consumed alcohol occasionally. No previous history<br />
of upper GI haemorrhage. Her regular medications were Co-Codamol,<br />
Alendronic acid, Lansoprazole, Oxybutynin, Gabapentin, GTN spray,<br />
Isosorbide mononitrate, Paroxetine, Diltiazem, Aspirin and Atorvastatin.<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
6<br />
Image [1] CT image on previous admission showing A-Abdominal<br />
Aortic Aneurysm (AAA) belly. B-Duodenum clinging around the aortic<br />
aneurysm, but no gas with in the aneurysm<br />
Image 1<br />
Three months prior to the last admission she presented to the ED after<br />
a fall and sustained a fracture dislocation of the left shoulder. A chest<br />
X-ray then showed a small density in the left upper zone measuring<br />
approximately 12mm. She underwent surgery for her fracture and was<br />
discharged from the hospital. An outpatient CT thorax and abdomen<br />
performed two months later to evaluate the lung mass incidentally<br />
revealed a 6.4 cm abdominal aortic aneurysm, which extended down<br />
to the bifurcation. The descending thoracic aorta was also aneurysmal<br />
[Image 1]. The mass in the lung was reported as possible fibrosis. She<br />
was referred to vascular surgeons for further management of AAA.<br />
A decision was made by the vascular surgery team not to intervene<br />
because of the morphology of aneurysm, as well as patient’s age,<br />
comorbidities and frailty.<br />
Blood results during the last admission with GI haemorrhage revealed<br />
an Hb of 94 g/L (112 g/L when discharged home from previous<br />
admission), white cell count of 34.1 x10^9/L (neutrophil count 30.8<br />
x10^9/L), platelet count of 352 x10^9/L, MCV of 90 fL. U&Es revealed<br />
Na-143 mmol/L, K-4.5 mmol/L, Urea 8.6 mmol/L, creatinine 95 umol/L,<br />
CRP-20 mg/L, normal LFTs and normal clotting parameters.
CASE REPORT<br />
You’ve probably<br />
never seen an oral iron<br />
like FERACCRU ® before<br />
For the treatment of adults with iron defi ciency<br />
anaemia (IDA) 1<br />
PRESCRIBING INFORMATION: Feraccru 30mg hard<br />
capsules (ferric maltol)<br />
Please refer to the full Summary of Product Characteristics<br />
(SmPC) before prescribing.<br />
Presentation: Red hard capsules. Each capsule contains<br />
30 mg iron (as ferric maltol). Indication: Feraccru is<br />
indicated in adults for the treatment of iron defi ciency.<br />
Dosage and Administration: Adults: Feraccru should be<br />
taken orally. The whole capsule should be taken on an<br />
empty stomach (with half a glass of water). The<br />
recommended dose is one capsule twice daily, in the<br />
morning and evening. The absorption of iron is reduced<br />
when Feraccru is taken with food. Treatment duration will<br />
depend on the severity of iron defi ciency but generally at<br />
least 12 weeks treatment is required. The treatment should<br />
be continued as long as necessary to replenish the body<br />
iron stores according to blood tests. Elderly: No dose<br />
adjustment is necessary. Children: The safety and effi cacy<br />
of Feraccru in children (17 years and under) has not yet<br />
been established. No data are available. Patients with<br />
hepatic or renal impairment: No clinical data is available<br />
in this patient population. Contraindications:<br />
Hypersensitivity to the active substance or to any of the<br />
excipients; Haemochromatosis and other iron overload<br />
syndromes; Patients receiving repeated blood<br />
transfusions. Warnings and precautions: Feraccru should<br />
not be used in patients with infl ammatory bowel disease<br />
(IBD) fl are or in IBD patients with haemoglobin (Hb) levels<br />
CASE REPORT<br />
Examination revealed HR-95 b/m, BP 130/70 mm Hg, oxygen saturation<br />
96% on 2 Litres of oxygen and normal temperature. Abdominal<br />
examination revealed a large pulsatile abdominal mass. PR examination<br />
revealed melaena on glove. She scored 10 points on the Glasgow-<br />
Blatchford Bleeding Score.<br />
After resuscitation with IV fluids and blood products the patient was<br />
transferred to the ward. She had 3 further large episodes of melaena<br />
on the same day and was taken to the endoscopy unit for upper GI<br />
endoscopy which did not reveal blood in the upper GI tract, but did<br />
show a deep ulcer in the second part of duodenum (D2) [Image 2&3].<br />
Post- endoscopy Rockall Score was 6. The consultant who performed<br />
the endoscopy was concerned about the morphology of the ulcer<br />
and recommended a CT scan and urgent surgical review to rule out<br />
a primary aorto-enteric fistula [PAEF]. In view of high suspicion of a<br />
PAEF, the endoscopist elected not to perform endotherapy used for<br />
bleeding duodenal ulcer. A 3 phase CT abdomen and pelvis revealed<br />
an increase in size of the infrarenal abdominal aortic aneurysm [AAA]<br />
which measured 8 cm [Image 4] (6.4cm previously [Image 1]). New<br />
gas locules within the inferior aspect of aneurysm sac were highly<br />
suggestive of aorto-enteric fistulation. The aortic sac was inseparable<br />
from the proximal duodenum and tracking gas locules were suggestive<br />
of a communication between aneurysm sac and second part of<br />
duodenum [Image 4]. An urgent vascular review was requested and<br />
vascular surgeons decided not to surgically intervene as the patient<br />
was very frail and unlikely to survive a repair. This was discussed with<br />
the patient and her daughter and decision for palliation was made. The<br />
patient had another massive haematemesis and melaena early in the<br />
morning of the next day and died after a few hours.<br />
Discussion<br />
Formation of fistula between aorta and intestinal tract was first described<br />
by an English Surgeon Sir Ashley Cooper in 1829 [1]. But the first case<br />
report of PAEF was published by Salmon in 1843. Since then only about<br />
400 of PAEF cases have been reported up until now [2&3]. PAEF is a<br />
potentially fatal and a rare complication of untreated aortic aneurysm,<br />
which warrants a high index of clinical suspicion when presented with<br />
upper GI haemorrhage. The fistula most commonly erodes into an<br />
area of intestine (most commonly the third part of duodenum) which<br />
has close contact with the pulsating aortic segment [4, 5&6]<br />
The classical triad of symptoms with abdominal pain, pulsatile<br />
abdominal mass and gastrointestinal haemorrhage are only found<br />
in 6-12% of patients [7&8]. The most common presentation is upper<br />
GI haemorrhage. Often the initial GI bleeding is small (herald bleed),<br />
but one third of the patients presents with massive recurrent bleeding<br />
within 6 hours [9]. Many patients with aorto-enteric fistula (AEF) die<br />
before an accurate diagnosis is made due to its variable clinical<br />
manifestations and insufficient awareness of this rare entity among<br />
non-specialty clinicians. Although literature review suggests the most<br />
common aetiology for PAEF are atherosclerotic and traumatic AAA,<br />
other causes include radiation, cancer, metastasis, gallstone erosion,<br />
ulcers, diverticulitis appendicitis, aortitis, infections like TB, pancreatic<br />
pseudo cyst penetration and foreign body ingestion[10&3].<br />
Unusual features of this case include the fistulous connection between<br />
the AAA and second part of duodenum and the presence of a deep<br />
ulcer on endoscopy. Even though no literature is available to confirm,<br />
it is possible that standard endotherapy for bleeding duodenal ulcer<br />
(adrenaline injection, heater probe, gold probe coagulation and<br />
endoclip placement) may lead to massive haemorrhage in patients<br />
with aorto-enteric fistula. In our patient, possibility of PAEF was<br />
considered very high and endotherapy was not attempted.<br />
Though less frequent, there are reports in literature that described<br />
abnormal communication between aorta and other pat of GI tract such<br />
as the oesophagus, jejunum, ileum and colon, but most of these were<br />
secondary to endovascular or other surgical procedures on aorta<br />
causing secondary fistulation [11].<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
Image [2] A-Endoscopic view of deep ulcer in D2<br />
Image [3] A-Endoscopic view of deep ulcer in D2<br />
Image 2<br />
8<br />
Image 3
Life feels good when they’re under control 1-8<br />
CASE REPORT<br />
CROHN’S DISEASE<br />
Indicated for the induction of<br />
remission in patients with mild to<br />
moderate active Crohn’s disease<br />
affecting the ileum and/or the<br />
ascending colon 9<br />
ULCERATIVE COLITIS<br />
Indicated for ulcerative<br />
colitis involving rectal and<br />
recto-sigmoid disease 10<br />
MICROSCOPIC<br />
COLITIS<br />
Indicated for the induction<br />
and maintenance of remission<br />
in patients with microscopic<br />
colitis 9<br />
Entocort ® CR is the ONLY<br />
LICENSED TREATMENT<br />
for the induction and<br />
maintenance of remission<br />
in microscopic colitis 9<br />
ENTOCORT CR 3mg Capsules (budesonide) - Prescribing Information<br />
Please consult the Summary of Product Characteristics (SmPC) for full<br />
prescribing Information<br />
Presentation: Hard gelatin capsules for oral administration with an opaque,<br />
light grey body and an opaque, pink cap marked CIR 3mg in black radial print.<br />
Contains 3mg budesonide. Indications: Induction of remission in patients with<br />
mild to moderate Crohn’s disease affecting the ileum and/or the ascending<br />
colon. Induction of remission in patients with active microscopic colitis.<br />
Maintenance of remission in patients with microscopic colitis. Dosage and<br />
administration: Active Crohn’s disease (Adults): 9mg once daily in the morning<br />
for up to eight weeks. Full effect achieved in 2-4 weeks. When treatment is to<br />
be discontinued, dose should normally be reduced in final 2-4 weeks. Active<br />
microscopic colitis (Adults): 9mg once daily in the morning. Maintenance of<br />
microscopic colitis (Adults): 6mg once daily in the morning, or the lowest<br />
effective dose. Paediatric population: Not recommended. Older people: No<br />
special dose adjustment recommended. Swallow whole with water. Do not<br />
chew. Contraindications: Hypersensitivity to the active substance or any of the<br />
excipients. Warnings and Precautions: Side effects typical of corticosteroids<br />
may occur. Visual disturbances may occur. If a patient presents with symptoms<br />
such as blurred vision or other visual disturbances they should be considered<br />
for referral to an ophthalmologist for evaluation of the possible causes.<br />
Systemic effects may include glaucoma and when prescribed at high doses for<br />
prolonged periods, Cushing’s syndrome, adrenal suppression, growth<br />
retardation, decreased bone mineral density and cataract. Caution in patients<br />
with infection, hypertension, diabetes mellitus, osteoporosis, peptic ulcer,<br />
glaucoma or cataracts or with a family history of diabetes or glaucoma.<br />
Particular care in patients with existing or previous history of severe affective<br />
disorders in them or their first degree relatives. Caution when transferring from<br />
glucocorticoid of high systemic effect to Entocort CR. Chicken pox and measles<br />
may have a more serious course in patients on oral steroids. They may also<br />
suppress the HPA axis and reduce the stress response. Reduced liver function<br />
may increase systemic exposure. When treatment is discontinued, reduce<br />
dose over last 2-4 weeks. Concomitant use of CYP3A inhibitors, such as<br />
ketoconazole and cobicistat-containing products, is expected to increase the<br />
risk of systemic side effects and should be avoided unless the benefits<br />
outweigh the risks. Excessive grapefruit juice may increase systemic exposure<br />
and should be avoided. Patients with fructose intolerance, glucose-galactose<br />
malabsorption or sucrose-isomaltase insufficiency should not take Entocort CR.<br />
Monitor height of children who use prolonged glucocorticoid therapy for risk of<br />
growth suppression. Interactions: Concomitant colestyramine may reduce<br />
Entocort CR uptake. Concomitant oestrogen and contraceptive steroids may<br />
increase effects. CYP3A4 inhibitors may increase systemic exposure. CYP3A4<br />
inducers may reduce systemic exposure. May cause low values in ACTH<br />
stimulation test. Fertility, pregnancy and lactation: Only to be used during<br />
pregnancy when the potential benefits to the mother outweigh the risks for the<br />
foetus. May be used during breast feeding. Adverse reactions: Common:<br />
Cushingoid features, hypokalaemia, behavioural changes such as nervousness,<br />
insomnia, mood swings and depression, palpitations, dyspepsia, skin reactions<br />
(urticaria, exanthema), muscle cramps, menstrual disorders. Uncommon:<br />
anxiety, tremor, psychomotor hyperactivity. Rare: aggression, glaucoma,<br />
cataract, blurred vision, ecchymosis. Very rare: Anaphylactic reaction, growth<br />
retardation. Prescribers should consult the summary of product characteristics<br />
in relation to other adverse reactions. Marketing Authorisation Numbers,<br />
Package Quantities and basic NHS price: PL 36633/0006. Packs of 100<br />
capsules: £84.15. Legal category: POM. Marketing Authorisation Holder:<br />
Tillotts Pharma UK Ltd, The Stables, Wellingore Hall, Wellingore, Lincoln, LN5<br />
0HX. Date of preparation of PI: November 2018<br />
ENTOCORT (budesonide) ENEMA - Prescribing Information<br />
Please consult the Summary of Product Characteristics (SmPC) for full<br />
prescribing Information<br />
Presentation: 0.02 mg/ml budesonide (2 mg budesonide/100 ml) solution for<br />
rectal suspension. Each Entocort Enema consists of 2 components: a 2.3 mg<br />
faintly yellow, circular biconvex tablet with the engraving BA1 on one side and<br />
2.3 on the other side; a 115 ml clear colourless solution. Indications: Ulcerative<br />
colitis involving rectal and recto-sigmoid disease. Dosage and administration:<br />
Route of administration: rectal. Adults: One Entocort Enema nightly for 4<br />
weeks. Full effect is usually achieved within 2–4 weeks. If the patient is not in<br />
remission after 4 weeks, treatment may be prolonged to 8 weeks. Paediatric<br />
population: Not recommended. Older people: Dosage as for adults. No dosage<br />
reduction in patients with reduced liver function. Instruct the patient to read the<br />
instructions for use. Reconstitute the enema immediately before use. Ensure<br />
the tablet is completely dissolved. Administer in the evening before bed.<br />
Contraindications: Hypersensitivity to the active substance or the excipients.<br />
Warnings and Precautions: Side effects typical of corticosteroids may occur,<br />
including glaucoma. Visual disturbances may occur. If a patient presents with<br />
symptoms such as blurred vision or other visual disturbances they should be<br />
considered for referral to an ophthalmologist for evaluation. When patients are<br />
transferred from steroids of higher systemic effect they may have adrenocortical<br />
suppression; monitoring may be considered and the dose of systemic steroid<br />
should be reduced cautiously. Replacement of high systemic effect steroid<br />
treatment with Entocort enema sometimes unmasks allergies which were<br />
previously controlled by the systemic drug. Reduced liver function affects the<br />
elimination of glucocorticosteroids, causing lower elimination rate and higher<br />
systemic exposure, with possible systemic side effects. Care when considering<br />
systemic corticosteroids in patients with existing or previous history of severe<br />
affective disorders in themselves or first degree relatives e.g. depressive or<br />
manic-depressive illness and previous steroid psychosis. Systemic effects<br />
of steroids may occur, particularly at high doses and for prolonged periods,<br />
including Cushing’s syndrome, adrenal suppression, growth retardation,<br />
decreased bone mineral density, cataract, glaucoma and very rarely a wide<br />
range of psychiatric/behavioural effects. Contains lactose and methyl-,<br />
propyl-parahydroxybenzoate. Caution in patients with hypersensitivity to<br />
these. Some patients may feel unwell in a non-specific way during withdrawal.<br />
When Entocort Enema is used chronically in excessive doses, systemic<br />
glucocorticosteroid effects may appear. However, the dosage form and the<br />
route of administration make any prolonged overdosage unlikely. Interactions:<br />
Raised plasma concentrations and enhanced effects of corticosteroids have<br />
been reported in women also treated with oestrogens and contraceptive<br />
steroids. Inhibitors of CYP3A4 can increase systemic exposure to budesonide<br />
several times and the combination should be avoided. If this is not possible, the<br />
period between treatments should as long as possible, and a reduction of the<br />
budesonide dose could also be considered. Other potent inhibitors of CYP3A4<br />
are also likely to markedly increase plasma levels of budesonide. Concomitant<br />
treatment with CYP3A4 inducers may reduce budesonide exposure and<br />
require a dose increase. Because adrenal function may be suppressed, an<br />
ACTH stimulation test for diagnosing pituitary insufficiency might show low<br />
values. Fertility, pregnancy and lactation: Only to be used during pregnancy<br />
when the potential benefits to the mother outweigh the risks for the foetus. May<br />
be used during breast feeding. Adverse reactions: Common: depression,<br />
gastrointestinal disturbances (flatulence, nausea, diarrhoea), skin reactions<br />
(urticaria, exanthema). Uncommon: agitation, insomnia, anxiety, psychomotor<br />
hyperactivity, duodenal or gastric ulcer. Rare: signs or symptoms of systemic<br />
glucocorticosteroid effects, aggression, glaucoma, cataract including<br />
subcapsular cataract, blurred vision, pancreatitis, ecchymosis, osteonecrosis.<br />
Very rare: anaphylactic reaction. Prescribers should consult the summary<br />
of product characteristics in relation to other adverse reactions. Marketing<br />
Authorisation Numbers, Package Quantities and basic NHS price: PL<br />
36633/0007. Packs of 7 enemas: £33.66. Legal category: POM. Marketing<br />
Authorisation Holder: Tillotts Pharma UK Ltd, The Stables, Wellingore Hall,<br />
Wellingore, Lincoln, LN5 0HX. Date of preparation of PI: March 2018<br />
Adverse events should be reported. Reporting forms and<br />
information can be found at https://yellowcard.mhra.gov.uk.<br />
Adverse events should also be reported to Tillotts Pharma<br />
UK Ltd. Tel: 01522 813500.<br />
References: 1. Greenberg GR et al. N Engl J Med 1994;331:836-841.<br />
2. Rezaie A et al. Cochrane Database Syst Rev 2015;6:CD000296.<br />
3. Madisch A et al. Int J Colorectal Dis 2005;20(4):312-316. 4. Hofer KN. Ann<br />
Pharmacother 2003;37:1457-1464. 5. Miehlke S et al. <strong>Gastroenterology</strong><br />
2008;135:1510-1516. 6. Gross V et al. Aliment Pharmacol Ther 2006;23:303-<br />
312. 7. Hartmann F et al. Aliment Pharmacol Ther 2010;32(3):368-376.<br />
8. Danielsson A et al. Scand J Gastroenterol 1992;27(1):9-12. 9. Entocort ® CR<br />
3mg Capsules – Summary of Product Characteristics. November 2018.<br />
10. Entocort ® Enema – Summary of Product Characteristics. March 2018.<br />
Date of<br />
preparation:<br />
December 2018.<br />
PU-00225.<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
9
CASE REPORT<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
10<br />
The diagnosis of aorto-enteric fistula typically is delayed, as long as 1<br />
month in 50% of patients in one series, with the diagnosis being made<br />
within 10 days of hospitalization in only 15% of cases [12]. Urgent upper<br />
GI endoscopy is important for exclusion of common causes of massive<br />
upper GI bleeding; but the diagnostic sensitivity for aorto-enteric fistula<br />
has been reported to be as low as 25% [12]. Gastrointestinal bleeding<br />
with endoscopically unclear findings in a patient with aortic aneurysm or<br />
history of aortic repair should points towards an aorto-enteric fistula [13].<br />
The most valuable tool for diagnosing AEF is a CT scan with contrast,<br />
which may reveal gas within the aneurysm, destruction of the fat plane<br />
between the aneurysm and duodenum, proximity and connection<br />
between aorta and intestine and leaking of the contrast into the GI lumen;<br />
all highly suggestive of AEF [14]. MRI is less useful because of its limited<br />
availability in the emergency setting, longer acquisition time, need for<br />
local technical expertise, and potential difficulties differentiating peri-graft<br />
gas from aortic wall calcification. All other investigation modalities like<br />
white blood cell scan are of limited value in diagnosing AEF. Percutaneous<br />
angiography has a sensitivity of 94 percent and a specificity of 85 percent<br />
for detecting aorto-enteric fistula (AEF); but is rarely considered as<br />
most patients are critically ill by the time of a decision is made to do it<br />
[15&16]. ]. A high index of suspicion is paramount, supplemented by the<br />
judicious use of upper endoscopy and CT, and the attending physician<br />
must be willing to recommend exploratory laparotomy if clinical suspicion<br />
is sufficiently high. As immediate and correct diagnosis is difficult, the<br />
mortality is very high and an untreated AEF has 100% mortality. Mortality<br />
of invasively treated patients is approximately 50 %. [17].<br />
Conclusion<br />
Although AEF is an extremely rare cause of upper GI haemorrhage, it<br />
must always be considered and ruled out in GI haemorrhages. A herald<br />
Image [4] Coronal reconstruction on this portal venous CT study<br />
demonstrates A-Abdominal Aortic Aneurysm (AAA) belly which has<br />
increased in size compared to previous CT image B-New gas locule<br />
within the inferior aspect of aneurysm sac C-Inseparable aortic sac<br />
from the proximal duodenum and tracking gas locules suggestive of a<br />
Image communication 4 between aneurysm sac and second part of duodenum<br />
bleed followed by a an endoscopy is usually the first step in diagnosis.<br />
This case highlights the importance of high index of suspicions in<br />
diagnosing AEF, especially if the patient is known to have AAA. PAEF<br />
is rare if the patient is not known to have AAA; but an accurate clinical<br />
evaluation to rule out an abdominal bruit or pulsatile mass should be<br />
done to rule out AEF. AEF is potentially fatal. An early diagnosis with<br />
prompt surgical management has huge prognostic benefits.<br />
References<br />
1. Cooper A. Lectures on the Principles and Practice of Surgery, London 1829<br />
2. Abdu Hassan Alzobydi and Shaista Salman Guraya. Primary aortoduodenal fistula: A case<br />
reportWorld J Gastroenterol. 2013 Jan 21; 19(3): 415–417.Published online 2013 Jan<br />
21. doi: 10.3748/wjg.v19.i3.415<br />
3. Daniele Bissacco, Luca Freni, Luca Attisani, Iacopo Barbetta, Raffaello Dallatana, and<br />
Piergiorgio Settembrini. Unusual clinical presentation of primaryaortoduodenal fistula.<br />
Gastroenterol Rep (oxf). 2015 may; 3(2): 170–174. Published online 2014 Jun 30. doi:<br />
10.1093/gastro/gou040<br />
4. Jarboui S, Jarraya H, Mahjoub W, Baccar M, Kacem C, Abdesselem MM, Zaouche A.<br />
Primary aorto-enteric fistula in a 52-year-old man. Tunis Med. 2008 Sep; 86(9):830-2.<br />
5. Debonnaire P, Van Rillaer O, Arts J, Ramboer K, Tubbax H, Van Hootegem P. Primary aorto<br />
enteric fistula: Report of 18 Belgian cases and literature review. Acta Gastroenterol Belg.<br />
2008 Apr-Jun; 71(2):250-8.<br />
6. O’Mara C, Imbembo AL. Paraprosthetic-enteric fistula. Surgery. 1977 May; 81(5):556-66.<br />
7. Calligaro KD, Bergen WS, Savarese RP, Westcott CJ, Azurin DJ, Delaurentis DA. Primary<br />
aortoduodenal fistula due to septic aortitis. J Cardiovasc Surg (Torino). 1992 Mar-Apr;<br />
33(2):192-8.<br />
8. Voorhoeve R,Moll FL, De letter JA, Bast TJ Wester JP, Slee PH. Primary aortoenteric<br />
fistula: Report of eight new cases and review of the literature. Ann Vasc Surg. 1996 Jan;<br />
10(1):40-8.<br />
9. Fernández de Sevilla E, Echeverri JA, Boqué M, Valverde S, Ortega N, Gené A, Rodríguez<br />
N, Balibrea JM, Armengol M. Life-threating upper gastrointestinal bleeding due to<br />
a primary aorto-jejunal fistula. Int J Surg Case Rep. 2015; 8C:25-8. doi: 10.1016/j.<br />
ijscr.2015.01.010. Epub 2015 Jan 9.<br />
10. Sharma K, Kibria R, Ali S, Rao P. Primary aortoenteric fistula caused by an infected<br />
abdominal aortic aneurysm with Mycobacterium avium complex in an HIV patient. Acta<br />
Gastroenterol Belg. 2010 Apr-Jun; 73(2):280-2.<br />
11. Sin-Jae Kang, Dong-LK, Kim, Se-Ho Huh, Byung-Boong Lee, Duk-Kyung Kim, and<br />
Young-Soo Do. Coexisting aortocolic and aortovesical fistulae in an abdominal aortic<br />
aneurysm: Report of a case. Surgery today. June 2003, Volume 33, Issue 6, pp 441–443.<br />
12. Pipinos II, Carr, J.A., Haithcock, B.E., Anagnostopoulos, P.V., Dossa, C.D., and Reddy, D.J.<br />
Secondary aorto-enteric fistula. Ann Vasc Surg. 2000; 14: 688–696).<br />
13. Franke S, Debus ES, Voit R. Aorto-intestinal fistula as a possible cause of endoscopically<br />
undetermined gastrointestinal hemorrhage. [Article in German]. Chirurg. 1995 Feb;<br />
66(2):112-9<br />
14. Saers SJ, Scheltinga MR. Diagnostic image (154). A man with haematemesis and gas in<br />
the aorta. Primary aorto-enteric fistula. [Article in Dutch]. Ned tijdschr geneeskd. 2003<br />
Aug 30; 147(35):1686<br />
15. Yeong KY. Angiographic demonstration of primary aorto-enteric fistula--A case report.<br />
Ann Acad Med Singapore. 1995 May; 24(3):467-9.<br />
16. Huges FM1, Kavanagh D Barry M, Owen A, Macerlaine DP, Malone de, Aortoenteric<br />
Fistula: a diagnostic dialemma. Abdom imaging.2007 May-Jun; 32(3):398-402.epub<br />
2006 Aug 25<br />
17. Behrendt CA, Wipper S, Debus SE, von Kodolitsch Y, Püschel K, Kammal M, Kammal<br />
A. Primary aorto-enteric fistula as a rare cause of massive gastrointestinal haemorrhage.<br />
Vasa. 2017 Oct; 46(6):425-430. doi: 10.1024/0301-1526/a000646. Epub 2017 Jun 30.<br />
Consent for Publication<br />
Written consent obtained<br />
Address for correspondence<br />
Dr Mohammed Shaheer Pandara Arakkal, Senior Clinical fellow in<br />
<strong>Gastroenterology</strong>, House G, Morriston Hospital, Swansea SA6 6NL
FEATURE<br />
INDEPENDENTLY<br />
SUPPORTED BY RESEARCH<br />
TO ARRIVE, SURVIVE, AND<br />
THRIVE IN THE GUT<br />
A unique, water-based<br />
formulation to help<br />
support the gut microbiome<br />
Its multi-strain bacteria arrives<br />
in a live and active state,<br />
ready to act in the gut<br />
Its water-based nature survives<br />
stomach acid, allowing the<br />
bacteria to reach the gut intact<br />
Bacteria are clinically proven<br />
to rapidly colonise in the<br />
gut, contributing to gut<br />
microbiome balance<br />
To find out more about Symprove TM contact<br />
01252 413 600 or support@symprove.com<br />
www.symprove.com/bioscience<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
References:<br />
1. Freuda-Agyeman, M et al. (2014). Comparative survival of commercial probiotic formulations: tests in biorelevant gastric fluids and real-time measurements using<br />
microcalorimetry. Beneficial Microbes. 6(1). 141-151<br />
2. Data on file. Results from a Symprove TM Customer Survey<br />
3. Wang, B et al. (2017). The human microbiota in health and disease. Engineering 3(1). 71-82<br />
4. Sisson G, Ayis S, Sherwood RA and Bjarnason I. (2014). Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome<br />
– a 12 week double-blind study. Aliment Pharmacol Ther 40. 51-62<br />
11
Dr. Daniel Vinteler<br />
CEO and Founder of Plasmabiotics, invented the PlasmaTYPHOON<br />
and PlasmaBAG; a device designed to minimize the risk of bacterial<br />
contamination in endoscopy. Daniel Vinteler has been in close contact<br />
with patients from the very beginning. This has provided him with a<br />
comprehensive understanding of the hygiene risks in endoscopy and<br />
this revolutionary new way to reduce them.<br />
‘Drying is a possible pitfall for hygiene<br />
in endoscopy’<br />
The diagnostic and therapeutic utility of Endoscopic Retrograde Cholangio-Pancreatography (ERCP) has been well demonstrated<br />
for a variety of disorders, such as the management and treatment of biliopancreatic diseases. Due to their complex design,<br />
duodenoscopes have been long recognized to require thorough processes and precise execution to properly disinfect.<br />
As the importance of ERCP procedures and their impact on patients’ lives remains unwavering. We speak to Dr. Daniel Vinteler<br />
(PlasmaBiotics) and Ulrike Beilenhoff (ESGENA) about their ideas concerning the challenges and solutions for the future of<br />
endoscopy and PlasmaBiotics latest technology, the PlasmaTYPHOON.<br />
How important is it to raise awareness<br />
about the risk of infection, during endoscopy<br />
procedures in a cross functional<br />
way?<br />
Ulrike Beilenhoff: Hygiene is an important<br />
concern as it relates to patient’s safety.<br />
Hospitals have the task to ensure a safe<br />
environment in order to prevent any adverse<br />
events and complications. The patient is in a<br />
vulnerable situation, they may have existing<br />
health problems, they are undergoing an<br />
endoscopy procedure, and when you expose<br />
the patient to additional germs, you create<br />
an additional risk. Since the early 2000s<br />
we are experiencing problems with multidrug<br />
resistant bacteria. If a patient is ill<br />
and subsequently exposed to this bacteria,<br />
hospitals have limited options to treat the<br />
patient. The aim is to avoid any additional risk<br />
and therefore hygiene is really a core focus in<br />
this commitment. Thus, establishing hygiene<br />
as a commitment to the patient’s safety.<br />
What are the hygiene issues inherent to<br />
endoscope handling and storage?<br />
Daniel Vinteler: Every step of endoscope<br />
reprocessing is crucial to ensure a hygienic<br />
procedure: the pre-cleaning, cleaningdisinfection,<br />
drying and storage. If one of<br />
these steps is not performed correctly,<br />
hygiene is not guaranteed. In practice, the<br />
drying of the endoscope is often underestimated<br />
and therefore a possible pitfall for<br />
hygiene and reprocessing steps. If the scope<br />
is not dried properly, all the steps before are<br />
undercut.<br />
Why is it so crucial to dry an endoscope<br />
after the device has been disinfected?<br />
Ulrike Beilenhoff: Publications showed that<br />
several outbreaks have been caused by<br />
insuffi cient dyring. If you do not clean the<br />
endoscope properly then you leave residual<br />
debris. If you do not dry the endoscope<br />
it creates a perfect environment to breed<br />
germs. Nurses are aware of both of these<br />
steps. A perfectly dried endoscope stops<br />
bacterial proliferation.<br />
What are the key challenges and<br />
constraints in endoscope drying and<br />
storage? Why are these steps sometimes<br />
not perfectly carried out?<br />
Ulrike Beilenhoff: Time constraints in hospitals<br />
present challenges to effective drying and<br />
storage. Surveys showed that nurses and<br />
reprocessing staff work under time pressure.<br />
Even though they are aware of the importance<br />
of thorough cleaning and drying, it can be<br />
challenging. Suffi cient time is the prerequisite<br />
for correct reprocessing of endoscopes and<br />
this also includes suffi cient time for drying<br />
before storage.<br />
It is the responsibility of hospitals as service<br />
providers to ensure a proper training for all<br />
staff involved in endoscope reprocessing.
advertorial<br />
Nurses are not the only individuals working<br />
in scope reprocessing. If staff are not trained<br />
to understand the endoscope channels in<br />
detail, they may struggle to effectively dry the<br />
endoscope.<br />
How does the PlasmaTYPHOON device<br />
completely dry an endoscope?<br />
Daniel Vinteler: The PlasmaTYPHOON offers<br />
a new way to dry and store the scope<br />
with plasma, thereby reducing the risk of<br />
contamination. The drying process with the<br />
PlasmaTYPHOON is managed by a patented<br />
curve of pressure. The unit uses a laminar<br />
fl ow to eliminate the water and a turbulent<br />
heated fl ow to dry the walls.<br />
At PENTAX Medical we are committed<br />
to addressing the hygiene challenges in<br />
endoscopy by offering solutions that minimize<br />
the risk of infections, improve clinical<br />
outcomes, enhance provider experience,<br />
and increase healthcare productivity. We<br />
are actively listening to our customers<br />
and stakeholders, so we understand the<br />
complexity of the procedures and the risk of<br />
infection.<br />
How does the system improve on<br />
existing solutions? What advantages<br />
can staff expect while using the<br />
PlasmaTYPHOON?<br />
Daniel Vinteler: The PlasmaTYPHOON is the<br />
fi rst solution to guarantee a dry endoscope<br />
in up to 5 minutes 1 (the drying time depends<br />
on the endoscope type), and storage up to<br />
31 days 2,3 in a fully controlled environment.<br />
The advantages for staff are numerous<br />
and include saving time and space, cost<br />
reductions, mobility of the scopes while they<br />
are stored in the PlasmaBAG, and of course<br />
an improved level of hygiene.<br />
What advantage do you foresee in a<br />
system such as the PlasmaTYPHOON<br />
and BAG, which dries the scope perfectly<br />
in an automatized way and stores the<br />
scope in a protected environment?<br />
Ulrike Beilenhoff: Hospitals need an easy and<br />
fast system to dry endoscopes. Often medical<br />
professionals cannot visibly see that the<br />
endoscope is wet, as the exterior appears dry,<br />
thus presenting a hygiene challenge.<br />
Daniel Vinteler: The PlasmaTYPHOON offers<br />
a solution to these challenges, by perfectly<br />
drying the endoscope and doing so in an<br />
easy and fast manner. The PlasmaTYPHOON<br />
is the most advantageous option for<br />
endoscope reprocessing as it completes the<br />
task quickly and easily, without compromising<br />
effectiveness.<br />
1. Evaluation of the efficacy of a drying unit for internal channels of endoscopes according to NF S98-030- Test Report by Biotech-Germande February 2015.<br />
2. Evaluation of the ability of a storage system (Plasmabiotics) to maintain the microbiological quality of heat sensitive endoscope. Report by Biotech-Germande April 2017.<br />
3. The maximum storage time may be subject to local regulations on endoscope storage. The country regulation can restrict the maximum storage time to 7 days. Please refer to the relevant regulations or recommendation of your country.<br />
Ulrike Beilenhoff,<br />
Scientifi c Secretary and former President of<br />
the European Society of <strong>Gastroenterology</strong> and<br />
Endoscopy Nurses and Associates (ESGENA).<br />
Ulrike has important insights concerning<br />
the hygiene challenge in endoscopy;<br />
specialized in endoscopy nursing, with 16<br />
years of experience as head nurse in German<br />
Endoscopy Departments.
FEATURE<br />
SHOWING THE TRUE VALUE OF<br />
PROBIOTICS – UNDERSTANDING<br />
THE SCIENCE AND HISTORY IS KEY<br />
Written by Mike Butler, CEO, Symprove<br />
Therapeutic use of probiotics isn’t new. The use of<br />
fermented foods to improve intestinal health has<br />
been recorded back thousands of years, a long time<br />
before there was an ounce of understanding of the gut<br />
microbiome and the importance of its managment. 1<br />
The Russian scientist Eli Metchnikoff (generally believed to be the<br />
‘father of probiotics’) first hypothesised that lactic acid bacteria<br />
provided either a type of protection from, or reversal of, intestinal<br />
auto-intoxication. He won the Nobel Prize in medicine in 1908 by<br />
demonstrating harmful microbes can be replaced by beneficial<br />
microbes to treat intestinal illnesses. His regimen became sour<br />
milk in the hope of populating his digestive tract with Bulgarian<br />
bacillus (Lactobacillus bulgaricus) – the first use of ‘probiotics’<br />
as a dietary supplement. Mehcnikoff laid the foundations for<br />
faecal transplantation, predicted the existence of bacterial<br />
translocation from the intestinal lumen into the bloodstream and<br />
lymphatic system and theories linking chronic inflammation with<br />
atherosclerosis. 2<br />
As our knowledge of the gut microbiome has grown, so has our<br />
scepticism of probiotics. Some of this scepticism is fair, some<br />
not so, as we bucket a group of probiotics which come in various<br />
different shapes and sizes and treat them as equal. This is just<br />
not the case as there are various formulations, bacterial strains,<br />
mode of delivery and impact on microbiota to consider. 3 So what<br />
do we need to do to change perceptions and show the value of<br />
probiotics?<br />
Firstly, we need to be brave like Mehcnikoff and explore the<br />
Health Diagnostics for Digestive Health Management<br />
Meet the experts at BSG<br />
on stand no. 42<br />
SEC Glasgow, 17 th -20 th June<br />
Faecal Immunochemical<br />
Testing (FIT)<br />
for Bowel Cancer Screening or<br />
Symptomatic Assessment<br />
2021.12.31<br />
H<br />
999123<br />
g<br />
Name<br />
80101274<br />
Date of Sample<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
Calprotectin Testing<br />
Point of Care and Remote<br />
Patient Management<br />
Solutions<br />
Therapeutic Drug<br />
Monitoring<br />
New Quantum Blue ®<br />
Rapid Test for anti-TNFα<br />
Therapeutic Drug Monitoring<br />
Tel: 023 8048 3000 | Web: www.alphalabs.co.uk<br />
M / F<br />
14<br />
DDH+BSG-<strong>2019</strong>_FINAL_13May19.indd 1 13/05/<strong>2019</strong> 16:30:55
FEATURE<br />
possibilities of human health beyond our existing beliefs. Our gut<br />
microbiota contains tens of trillions of micro-organisms, including<br />
at least 1,000 different species of known bacteria with more than<br />
3 million genes (150 times more than human genes). To put it in<br />
perspective, the Human Genome Project took 15 years to complete<br />
so we inevitably have far more to understand about the makeup<br />
of the gut microbiome. However, already we are seeing positive<br />
results for some probiotics in improving people’s health. It will take<br />
time to know the exact potential of probiotics work and also our<br />
understanding of the gut microbiome, its full role and where it can<br />
be adapted to improve lives, but we are working with safe food<br />
supplements which allow us to be braver with their application.<br />
LUTATHERA ® ▼ LUTETIUM<br />
( 177 Lu) OXODOTREOTIDE<br />
Secondly, the balance between science driven and commercial<br />
goals needs to be heavily weighted towards the former. As<br />
probiotics are a safe food supplement, it is easy for some to focus<br />
on market penetration and not mode of action. However, if we are<br />
going to realise the potential of probiotics, an evidence based,<br />
long-term approach needs to be undertaken for all manufacturers<br />
of probiotics.<br />
By following the science, there is a real possibility that we might<br />
find something that is ‘game-changing’. This is already a growing<br />
appetite to push our current perceptions, understanding and<br />
application of probiotics. For example, trials are now looking at the<br />
link between the microbiome Parkinson’s and the gut brain-axis. 4<br />
As we continue to learn about the different ‘good’ bacteria,<br />
the way they arrive in the gut in an alive state, survive hostile<br />
gastric conditions and thrive in the colon, we will get a better<br />
understanding of this complex microsystem that is living inside all<br />
of us.<br />
Ten years from now, I am confident that we will be looking back<br />
at today as the cusp of a revolution, one in which the science-led<br />
probiotics will have an important part to play.<br />
References:<br />
1. Ozen M and Dinleyici EC. The history of probiotics: the untold<br />
story. Benef Microbes. 2015;6(2):159-65.<br />
2. Mackowiak PA. Recycling Metchnikoff: Probiotics, the Intestinal<br />
Microbiome and the Quest for Long Life. Front Public Health.<br />
2013; 1: 52.<br />
3. Gaisford S. et al. Comparative survival of commercial probiotic<br />
formulations: Tests in biorelevant gastric fluids and real-time<br />
measurements using microcalorimetry. Beneficial Microbes<br />
6(1): 1-11 · October 2014<br />
4. Chaudhuri R. Kings Colledge London. Oral Symprove (a<br />
probiotic) for the management of motor and non-motor<br />
symptoms in people with Parkinson’s: a novel randomised,<br />
double-blind, placebo-controlled pilot study.<br />
Lutathera ® ▼ lutetium ( 177 Lu) oxodotreotide is indicated for<br />
the treatment of unresectable or metastatic, progressive,<br />
well differentiated (G1 and G2), somatostatin receptor<br />
positive gastroenteropancreatic neuroendocrine tumours<br />
(GEP NETs) in adults.<br />
www.lutathera.com<br />
Adverse events should be reported. Reporting forms and information can be found at<br />
www.mhra.gov.uk/yellowcard.<br />
Adverse events should also be reported to Advanced Accelerator Applications at:<br />
pharmacovigilance@adacap.com<br />
pv@imagingequipment.co.uk<br />
Tel: 33 (0) 785 61 90 66, Switchboard +334 50 99 30 70, Fax +334 50 99 30 71<br />
Prescribing Information; Lutathera 370 MBq/mL solution for infusion. Lutetium ( 177 Lu)<br />
oxodotreotide Presentation: Solution for infusion. Clear, colourless to slightly yellow solution. One<br />
mL of solution contains 370 MBq of lutetium ( 177 Lu) oxodotreotide at the date and time of calibration.<br />
The total amount of radioactivity per single dose vial is 7,400 MBq at the date and time of infusion.<br />
Uses: Lutathera is indicated for the treatment of unresectable or metastatic, progressive, well<br />
differentiated (G1 and G2), somatostatin receptor positive gastroenteropancreatic neuroendocrine<br />
tumours (GEP NETs) in adults. Administration: Lutathera should be administered only by persons<br />
authorised to handle radiopharmaceuticals in designated clinical settings and after evaluation of<br />
the patient by a qualified physician. Before starting treatment with Lutathera, somatostatin receptor<br />
imaging (scintigraphy or positron emission tomography [PET]) must confirm the overexpression of<br />
these receptors in the tumour tissue with the tumour uptake at least as high as normal liver uptake<br />
(tumour uptake score ≥ 2). Additionally before each administration and during the treatment, tests are<br />
required to re assess the patient’s condition and adapt the therapeutic protocol if necessary (dose,<br />
infusion interval, number of infusions). See SmPC for further details. The recommended treatment<br />
regimen of Lutathera in adults consists of 4 infusions of 7,400 MBq each. The recommended interval<br />
between each administration is 8 weeks which could be extended up to 16 weeks in case of dose<br />
modifying toxicity (DMT). For renal protection purpose, an amino acid solution must be administered<br />
intravenously. See SmPC for further details. Given the fixed volumetric activity of 370 MBq/mL at the<br />
date and time of calibration, the volume of the solution is adjusted between 20.5 mL and 25.0 mL<br />
in order to provide the required amount of radioactivity at the date and time of infusion. Lutathera<br />
must be administered by slow intravenous infusion over approximately 30 minutes, concomitantly<br />
with amino acid solution administered by contralateral intravenous infusion (separate intravenous<br />
catheter and initiated 30 minutes prior to Lutathera). This medicinal product must not be injected<br />
as a bolus. Premedication with antiemetics should be injected 30 minutes before the start of amino<br />
acid solution infusion. The recommended infusion method for administration of Lutathera is the<br />
gravity method. During the administration the recommended precaution measures should be<br />
undertaken. Lutathera should be infused directly from its original container. The vial must not be<br />
opened or the solution transferred to another container. During the administration only disposable<br />
materials should be used. The medicinal product should be infused through an intravenous catheter<br />
placed in the vein exclusively for its infusion. See SmPC for further details of storage, room and<br />
equipment requirements, as well as detailed administration procedure. In some circumstances,<br />
it might be necessary to temporarily discontinue treatment with Lutathera, adapt the dose after<br />
the first administration or discontinue the treatment. General Warnings: Contraindications<br />
include hypersensitivity to the active substance, to any of the excipients, established or suspected<br />
pregnancy or when pregnancy has not been excluded, kidney failure with creatinine clearance<br />
< 30 mL/min. Special precautions should be taken where patients have renal or urinary tract<br />
morphological abnormalities, urinary incontinence, mild to moderate chronic kidney disease with<br />
creatinine clearance ≥ 50 mL/min, haematologic toxicity greater or equal to grade 2 (CTCAE) before<br />
treatment other than lymphopenia, bone metastasis or have had previous chemotherapy. Late-onset<br />
myelodisplastic syndrome (MDS) and acute leukaemia (AL) have been observed after treatment with<br />
Lutathera, factors such as age >70 years, impaired renal function, baseline cytopenias, prior number<br />
of therapies, prior exposure to chemotherapeutic agents (specifically alkylating agents), and prior<br />
radiotherapy are suggested as potential risks and/or predictive factors. Crises due to excessive<br />
release of hormones or bioactive substances may occur and overnight hospitalisation should be<br />
considered for vulnerable patients. Radioprotection rules should be followed including special<br />
care in the event of extravasation and urinary incontinence, see SmPC for full details or radio<br />
protective measures. The product contains up to 3.5 mmol sodium and this should be considered<br />
in patients on a sodium controlled diet. Undesirable effects: Common side effects include bone<br />
marrow toxicity with thrombocytopenia, lymphopenia, anaemia<br />
or pancytopenia. Nephrotoxicity with haematuria, renal failure,<br />
proteinuria. Blood creatinine increased, nausea, vomiting,<br />
fatigue, QT prolonged, hypertension, flushing, hypotension,<br />
dyspnoea, abdominal distension, diarrhoea, abdominal pain,<br />
constipation, dyspepsia, gastritis, hyperbilirubinaemia, alopecia,<br />
musculoskeletal pain, muscle spasms, acute kidney injury,<br />
increased LFTs. Marketing Authorisation Holder: Advanced<br />
Accelerator Applications 20 rue Diesel 01630 Saint Genis Pouilly<br />
France Marketing Authorisation Number EU/1/17/1226/001<br />
Legal Category: POM Price: £17,875 per vial Date of<br />
preparation of PI: October 2017<br />
Date of preparation: May <strong>2019</strong><br />
Job ID Number: AAA.Lu177-UKI-0013<br />
AAA-Lu177-GL-0028<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
15
From Norgine, the company that brought you<br />
MOVIPREP<br />
NEWS<br />
® (Macrogol 3350, sodium sulphate, ascorbic acid, sodium ascorbate and electrolytes)<br />
The first 1 litre PEG bowel preparation 1-3<br />
Improve the efficacy<br />
cut the volume<br />
vs MOVIPREP ®1<br />
PLENVU® PM/AM dosing was<br />
superior to MOVIPREP® in the per<br />
protocol population (successful<br />
overall cleansing 97.3% vs 92.2%,<br />
p=0.014)<br />
Safety profile comparable to<br />
MOVIPREP® 1,4-6<br />
Powder for Oral Solution<br />
PEG 3350, Sodium Ascorbate, Sodium<br />
Sulfate, Ascorbic Acid, Sodium Chloride,<br />
and Potassium Chloride<br />
UK AND IE PRESCRIBING INFORMATION: Plenvu powder for oral solution<br />
(Macrogol 3350 + Sodium ascorbate + Ascorbic acid + Sodium sulfate<br />
anhydrous + Potassium chloride + Sodium chloride)<br />
Please refer to the full Summary of Product Characteristics (SmPC)<br />
before prescribing.<br />
Presentation: Plenvu powder for oral solution is administered in two doses. Dose<br />
one is made up of 1 sachet containing: macrogol 3350 100g, sodium sulfate<br />
anhydrous 9g, sodium chloride 2g, potassium chloride 1g. Dose 2 is made up of<br />
2 sachets (A and B). Sachet A contains: macrogol 3350 40g, sodium chloride 3.2g,<br />
potassium chloride 1.2g. Sachet B contains: sodium ascorbate 48.11g, ascorbic<br />
acid 7.54g.<br />
Indication: For bowel cleansing in adults, prior to any procedure requiring a clean<br />
bowel.<br />
Dosage and administration: For oral use. Adults and elderly: A course of<br />
treatment consists of two separate non-identical 500ml doses of Plenvu. At least<br />
500ml of additional clear fluid must be taken with each dose. Treatment can be<br />
taken according to a two-day or one-day dosing schedule. Two-day dosing schedule:<br />
First dose taken the evening before the procedure. Second dose in the early morning<br />
of the day of the procedure. Morning only dosing schedule: Both doses taken the<br />
morning of the procedure. The two doses should be separated by a minimum of<br />
1 hour. Day before dosing schedule: Both doses taken the evening before the<br />
procedure. The two doses should be separated by a minimum of 1 hour. No solid<br />
food should be taken from the start of the course of treatment until after the clinical<br />
procedure. Consumption of all fluids should be stopped at least 2 hours prior to a<br />
procedure under general anaesthesia or 1 hour prior to a procedure without general<br />
anaesthesia. Children: Not recommended for use in children below 18 years of<br />
age. Patients with renal or hepatic impairment: No special dosage adjustment<br />
is deemed necessary in patients with mild to moderate renal or hepatic impairment.<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
Patients should be advised to allow adequate time after bowel movements have<br />
subsided to travel to the clinical unit.<br />
Contraindications: Hypersensitivity to the active substances or to any of the<br />
excipients, gastrointestinal obstruction or perforation, ileus, disorders of gastric<br />
emptying (gastroparesis, gastric retention), phenylketonuria, glucose-6-phosphate<br />
dehydrogenase deficiency, toxic megacolon.<br />
Warnings and precautions: The fluid content of reconstituted Plenvu does not<br />
replace regular fluid intake. Adequate fluid intake must be maintained. As with<br />
other macrogol containing products, allergic reactions including rash, urticaria,<br />
pruritus, angioedema and anaphylaxis are a possibility. Caution should be used<br />
with administration to frail or debilitated patients, in patients with impaired gag<br />
reflex, with the possibility of regurgitation or aspiration, or with diminished levels<br />
of consciousness, severe renal impairment, cardiac failure (grade III or IV of NYHA),<br />
those at risk of arrhythmia, dehydration or severe acute inflammatory bowel disease.<br />
16<br />
In debilitated fragile patients, patients with poor health, those with clinically significant<br />
renal impairment, arrhythmia and those at risk of electrolyte imbalance, the physician<br />
should consider performing a baseline and post-treatment electrolyte, renal function<br />
test and ECG as appropriate.<br />
Any suspected dehydration should be corrected for before use of Plenvu.<br />
There have been rare reports of serious arrhythmias including atrial fibrillation<br />
associated with the use of ionic osmotic laxatives for bowel preparation, predominantly<br />
in patients with underlying cardiac risk factors and electrolyte disturbance.<br />
If patients develop any symptoms indicating arrhythmia or shifts of fluid/electrolytes<br />
during or after treatment, plasma electrolytes should be measured, ECG monitored<br />
and any abnormality treated appropriately.<br />
If patients experience severe bloating, abdominal distension, or abdominal pain,<br />
administration should be slowed or temporarily discontinued until the symptoms<br />
subside.<br />
The sodium content, 458.5mmol (10.5g), should be taken into consideration for<br />
patients on a controlled sodium diet. The potassium content, 29.4mmol (1.1g), should<br />
be taken into consideration by patients with reduced kidney function or those on a<br />
controlled potassium diet.<br />
Interactions: Medicinal products taken orally within one hour of starting colonic<br />
lavage with Plenvu may be flushed from the gastrointestinal tract unabsorbed. The<br />
therapeutic effect of drugs with a narrow therapeutic index or short half-life may be<br />
particularly affected.<br />
Fertility, pregnancy and lactation: There are no data on the effects of Plenvu on<br />
fertility in humans. There were no effects on fertility in studies in male and female rats.<br />
It is preferable to avoid the use of Plenvu during pregnancy.<br />
It is unknown whether Plenvu active ingredients/metabolites are excreted in human<br />
milk. A risk to the newborns/infants cannot be excluded. A decision must be made<br />
whether to discontinue breast-feeding or to abstain from Plenvu therapy taking into<br />
account the benefit of breast-feeding for the child and the benefit of therapy for<br />
the woman.<br />
Effects on ability to drive and use machines: Plenvu has no influence on the ability<br />
to drive and use machines.<br />
Undesirable effects: Diarrhoea is an expected outcome.<br />
Common: vomiting, nausea, dehydration. Uncommon: abdominal distension,<br />
anorectal discomfort, abdominal pain, drug hypersensitivity, headache, migraine,<br />
somnolence, thirst, fatigue, asthenia, chills, pains, aches, palpitation, sinus<br />
tachycardia, transient increase in blood pressure, hot flush, transient increase in<br />
liver enzymes, hypernatraemia, hypercalcaemia, hypophosphataemia, hypokalaemia,<br />
decreased bicarbonate, anion gap increased/ decreased, hyperosmolar state.<br />
Refer to the SmPC for a full list and frequency of adverse events.<br />
UNITED KINGDOM:<br />
Price and pack sizes: £12.43 (3 sachet)<br />
Legal category: Pharmacy medicine<br />
MA Number: PL 20011/0040<br />
MA Holder: Norgine Pharmaceuticals Limited, Norgine House, Widewater Place,<br />
Moorhall Road, Harefield, Uxbridge, UB9 6NS, UK<br />
IRELAND<br />
Legal category: Product subject to medical prescription which may be renewed<br />
MA Number: PA 1336/005/001<br />
MA Holder: Norgine BV, Hogehilweg 7, 1101CA Amsterdam ZO, The Netherlands<br />
Additional information is available on request or in the SmPC. For further<br />
information contact: Norgine Pharmaceuticals Limited, Norgine House,<br />
Moorhall Road, Harefield, Middlesex, United Kingdom UB9 6NS. Telephone:<br />
+44(0)1895 826606. Email: medinfo@norgine.com<br />
Date of preparation: March <strong>2019</strong><br />
Company reference: UK/PLV/0319/0147<br />
United Kingdom<br />
Adverse events should be reported. Reporting forms and<br />
information can be found at www.mhra.gov.uk/yellowcard.<br />
Adverse events should also be reported<br />
to Norgine Pharmaceuticals Ltd on:<br />
Tel: +44 (0)1895 826 606 Email: medinfo@norgine.com<br />
References: 1. Bisschops R, et al. Endoscopy 2018; doi: 10.1055/a-0638-8125. 2. Schreiber S, et al. Endoscopy 2018; doi: 10.1055/a-0639-5070. 3. DeMicco MP, et al. Gastrointest Endosc 2018; doi: 10.1016/j.gie.2017.07.047. 4. PLENVU ® UK Summary of Product<br />
Characteristics. October 2018. 5. MOVIPREP ® UK Summary of Product Characteristics. September 2018. 6. MOVIPREP ® Orange UK Summary of Product Characteristics. August 2017.<br />
UK/PLV/0519/0164<br />
Date of preparation: May <strong>2019</strong><br />
Ireland<br />
Healthcare professionals are asked to report any suspected<br />
adverse reactions via HPRA Pharmacovigilance, Earlsfort<br />
Terrace, IRL - Dublin 2; Tel: +353 1 6764971;<br />
Fax: +353 1 6762517.<br />
Website: www.hpra.ie; E-mail: medsafety@hpra.ie.<br />
Adverse events should also be reported to Norgine<br />
Pharmaceuticals Ltd on:<br />
Tel: +44 (0)1895 826 606 Email: medinfo@norgine.com
Moviprep and Moviprep Orange (Macrogol 3350, sodium sulphate,<br />
ascorbic acid, sodium ascorbate and electrolytes) Prescribing<br />
Information<br />
REFER TO THE SUMMARY OF PRODUCT CHARACTERISTICS (SmPC)<br />
BEFORE PRESCRIBING<br />
Presentation: A box containing two transparent bags, each containing<br />
two separate sachets, A and B. Sachet A contains macrogol 3350 100g;<br />
sodium sulphate anhydrous 7.5g; sodium chloride 2.691g and potassium<br />
chloride 1.015g as white to yellow powder. Sachet B contains ascorbic acid<br />
4.7g and sodium ascorbate 5.9g as white to light brown powder. Moviprep<br />
also contains aspartame (E951), acesulfame potassium (E950) and a lemon<br />
or orange flavour. Uses: Bowel cleansing prior to any clinical procedure<br />
requiring a clean bowel. Dosage and administration: Adults and Older<br />
People: A course of treatment consists of two litres of Moviprep. A litre of<br />
Moviprep consists of one Sachet A and one Sachet B dissolved together<br />
in water to make one litre. This one litre reconstituted solution should be<br />
drunk over a period of one to two hours. This process should be repeated<br />
with a second litre of Moviprep to complete the course. A further litre of<br />
clear fluid is recommended during the course of treatment. This course of<br />
treatment can be taken either as divided or as single doses and timing is<br />
dependent on whether the clinical procedure is conducted with or without<br />
general anaesthesia as specified below: For procedures conducted<br />
under general anaesthesia: 1. Divided doses: one litre of Moviprep in<br />
the evening before and one litre of Moviprep in the early morning of the<br />
day of the clinical procedure. Ensure consumption of Moviprep as well as<br />
any other clear fluids has finished at least two hours before the start of the<br />
clinical procedure. 2. Single dose: two litres of Moviprep in the evening<br />
before the clinical procedure or two litres of Moviprep in the morning of the<br />
clinical procedure. Ensure consumption of Moviprep as well as any other<br />
clear fluids has finished at least two hours before the start of the clinical<br />
procedure. For procedures conducted without general anaesthesia:<br />
1. Divided doses: one litre of Moviprep in the evening before and one litre of<br />
Moviprep in the early morning of the day of the clinical procedure. Ensure<br />
consumption of Moviprep as well as any other clear fluids has finished at<br />
least one hour before the start of the clinical procedure. 2. Single dose: two<br />
litres of Moviprep in the evening before the clinical procedure or two litres<br />
of Moviprep in the morning of the clinical procedure. Ensure consumption<br />
of Moviprep has finished at least two hours before the start of the clinical<br />
procedure. Ensure consumption of any clear fluids has finished at least<br />
one hour before the clinical procedure. Patients should be advised to<br />
allow for appropriate time to travel to the colonoscopy unit. No solid food<br />
should be taken from the start of the course of treatment until after the<br />
clinical procedure. Children: Not recommended in children below 18 years<br />
of age. Contra-indications, warnings etc: Contra-indications: Known<br />
or suspected hypersensitivity to any of the ingredients, gastrointestinal<br />
obstruction or perforation, disorders of gastric emptying, ileus,<br />
phenylketonuria, glucose-6-phosphate dehydrogenase deficiency, toxic<br />
megacolon which complicates very severe inflammatory conditions of the<br />
intestinal tract. Do not use in unconscious patients. Warnings: Diarrhoea is<br />
an expected effect. Administer with caution to fragile patients in poor health<br />
or patients with serious clinical impairment such as impaired gag reflex,<br />
or with a tendency to aspiration or regurgitation, impaired consciousness,<br />
severe renal insufficiency, cardiac impairment (NYHA grade III or IV), those at<br />
risk of arrhythmia, dehydration, severe acute inflammatory bowel disease.<br />
Dehydration, if present, should be corrected before using Moviprep. The<br />
reconstituted Moviprep does not replace regular fluid intake and adequate<br />
fluid intake must be maintained. Semi-conscious patients or patients prone<br />
to aspiration should be closely monitored during administration, particularly<br />
if this is via a naso-gastric route. If symptoms indicating arrhythmia or shifts<br />
of fluid or electrolytes occur, plasma electrolytes should be measured,<br />
ECG performed and any abnormality treated appropriately. In debilitated<br />
fragile patients, patients with poor health, those with clinically significant<br />
renal impairment, arrhythmia and those at risk of electrolyte imbalance,<br />
the physician should consider performing baseline and post-treatment<br />
electrolyte, renal function test and ECG as appropriate. The possibility<br />
of serious arrhythmias, predominantly in those with underlying cardiac<br />
risk factors and electrolyte disturbance cannot be ruled out. If patients<br />
experience symptoms which make it difficult to continue the preparation,<br />
they may slow down or temporarily stop consuming the solution and should<br />
consult their doctor. Moviprep containing orange flavour is not recommended<br />
for patients with glucose and galactose malabsorption. Moviprep contains<br />
56.2 mmol of absorbable sodium per litre (caution in patients on a<br />
controlled sodium diet), 14.2 mmol potassium per litre (caution in patients<br />
with reduced kidney function or patients on a controlled potassium diet).<br />
Interactions: Oral medication should not be taken within one hour of<br />
administration as it may be flushed from the GI tract and not absorbed.<br />
Pregnancy and lactation: There is no experience of use in pregnancy or<br />
lactation so it should only be used if judged essential by the physician. Side<br />
Effects: Very common or common: abdominal pain, nausea, abdominal<br />
distension, anal discomfort, malaise, pyrexia, vomiting, dyspepsia, hunger,<br />
thirst, sleep disorder, headache, dizziness, and rigors. Uncommon or<br />
unknown: Dysphagia, discomfort, abnormal liver function tests, allergic<br />
reactions including rash, urticaria, pruritus, erythema, angioedema and<br />
anaphylaxis, dyspnoea, electrolyte disturbances, dehydration, convulsions<br />
associated with severe hyponatraemia, transient increase in blood pressure,<br />
arrhythmia, palpitations, flatulence and retching. Refer to the Summary of<br />
Product Characteristics (SmPC) for full list and frequency of adverse events.<br />
Overdose: In case of gross accidental overdosage, conservative measures<br />
are usually sufficient. In the rare event of severe metabolic derangement,<br />
intravenous rehydration may be used. Pharmaceutical Particulars:<br />
Sachets: Store in the original package below 25°C. Reconstituted solution:<br />
Keep covered. May be stored for up to 24 hours below 25°C or in a<br />
refrigerator. Legal Category: UK – Pharmacy only, Ireland - Prescription<br />
medicine. Packs: One pack of Moviprep or Moviprep Orange contains a<br />
single treatment. Basic NHS Price: UK £10.36, Ireland €13.26 Marketing<br />
Authorisation Number: UK: PL 20142/0005 (Moviprep), PL 20011/0006<br />
(Moviprep Orange). IE: PA 1336/1/1(Moviprep), PA 1336/1/2 (Moviprep<br />
Orange). For further information contact: Norgine Pharmaceuticals Ltd,<br />
Moorhall Road, Harefield, Middlesex UB9 6NS Tel: +44 (0) 1895 826606<br />
E-mail: medinfo@norgine.com Date of preparation/revision: March 2018.<br />
Ref UK/MPR/0318/0182<br />
United Kingdom Adverse events should be reported. Reporting forms<br />
and information can be found at www.mhra.gov.uk/yellowcard.<br />
Adverse events should also be reported to Medical Information at<br />
Norgine Pharmaceuticals Ltd on 01895 826606.<br />
Ireland Healthcare professionals are asked to report any suspected<br />
adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace,<br />
IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517.<br />
Website: www.hpra.ie; E-mail: medsafety@hpra.ie.<br />
Norgine Adverse events should also be reported to<br />
Medical Information at Norgine Pharmaceuticals on +44 1895 826606<br />
or E-mail: medinfo@norgine.com<br />
PLENVU, MOVIPREP, NORGINE and<br />
the sail logo are registered trademarks<br />
of the Norgine group of companies.<br />
NVU UK IRE GASTRO TODAY JUNE AD 210x297_FINAL.indd 23/05/<strong>2019</strong> 11:11 2<br />
Raising the bar for IBD<br />
care - launch of <strong>2019</strong> IBD<br />
Standards<br />
By Rukshana Kapasi, Chair of IBD UK<br />
The <strong>2019</strong> BSG conference sees the launch<br />
of the revised UK-wide IBD Standards, new<br />
exciting guidelines which could lead to<br />
drastic changes in how we care for people<br />
with Crohn’s or Colitis.<br />
These build on the previous IBD Standards<br />
(published in 2009 and updated in 2013)<br />
and have been devised by IBD UK, a<br />
partnership of 17 patient and professional<br />
organisations, including the British Society of<br />
<strong>Gastroenterology</strong>, Royal College of Nursing,<br />
Crohn’s & Colitis UK, CIRCA and Ileostomy<br />
Association, who are working together for<br />
everyone affected by Inflammatory Bowel<br />
Disease.<br />
The first audit of IBD Services in 2006<br />
highlighted wide variation in the quality and<br />
consistency of care for people with Crohn’s<br />
and Colitis, and whilst significant improvements<br />
have been made, this is unfortunately still the<br />
case. Not only this, the way we care for people<br />
with Crohn’s and Colitis has changed in the<br />
intervening years, with new therapy options, and<br />
a shift to self-management and personalised<br />
care and support.<br />
This version of the standards has seen a<br />
transformation, putting the patient at the centre<br />
and ensuring that everything we are asking for<br />
will ultimately make a difference to people with<br />
the conditions.<br />
When revising the standards, it was also<br />
important for us to consider technological<br />
advances, which have led to a huge change<br />
in the way we treat patients. Alongside this,<br />
society now takes a more holistic view towards<br />
care, not just for people with Crohn’s and<br />
Colitis, but across condition areas.<br />
The <strong>2019</strong> IBD Standards are a framework<br />
of statements that define what should be<br />
put in place to deliver safe, high-quality,<br />
personalised care for people with Crohn’s and<br />
Colitis. They cover all stages of the patient<br />
journey: pre-diagnosis, newly diagnosed,<br />
flare management, surgery, inpatient care and<br />
ongoing care, and how the IBD service should<br />
NEWS<br />
be organised to deliver this. The Standards<br />
complement the recently published BSG and<br />
ACPGBI IBD guidelines.<br />
All IBD Services should be working towards<br />
delivering the IBD Standards and the IBD UK<br />
website will house guidance and resources<br />
to help services implement these. Also being<br />
launched is a benchmarking tool which<br />
will allow the IBD Service to compare its<br />
performance against the standards, as well as<br />
with other services.<br />
It is important to note that benchmarking is an<br />
extremely positive tool for IBD Services – as<br />
well as highlighting the things they are doing<br />
brilliantly at; it can be an opportunity to push<br />
through some real changes. It is designed<br />
to help with putting together business cases<br />
based on empirical evidence, to make the best<br />
use of resources and establish priorities.<br />
From the launch date in June to August, one<br />
person from the IBD Service (representing<br />
the team) can register to sign up the<br />
benchmarking tool on the IBD UK website. The<br />
period of September to December is when the<br />
tool will need to be filled out. This will involve<br />
answering questions that align with the IBD<br />
Standards statements.<br />
Alongside this, IBD UK, supported by Crohn’s<br />
& Colitis UK, will be launching the first UK-wide<br />
IBD patient survey to ensure that the patient<br />
perspective is central, and that the patient<br />
experience helps shape the IBD service.<br />
People with Crohn’s and Colitis will be able to<br />
anonymously feedback on the Service.<br />
The benchmarking tool together with the<br />
patient survey are valuable tools that will<br />
enable IBD Services to be more effective<br />
with their team and efficient with resources<br />
and finances. This is an exciting and ground<br />
breaking opportunity for services and patients<br />
to work collaboratively leading to the shared<br />
priority of improving the lives of people with<br />
Crohn’s and Colitis.<br />
The Standards have been produced by<br />
patients and professionals working together<br />
and it is fundamental that this partnership<br />
approach continues to make them a reality.<br />
We need everybody to get involved to<br />
make a difference. To find out more about<br />
the IBD Standards and to register for the<br />
benchmarking tool visit ibduk.org.uk<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
17
NEWS<br />
ARE YOU DOING YOUR BEST<br />
FOR PBC PATIENTS?<br />
Inadequate or intolerant response to UDCA is defined as<br />
ALP >1.67 x ULN (>200 IU/L) and/or bilirubin 2 x ULN 1<br />
1<br />
PBC expertise is closer<br />
than you think...<br />
Consider referral to 1 of 26<br />
PBC hubs in the UK comprised<br />
of specialist multidisciplinary<br />
teams for individualised patient<br />
treatment and second line therapy.<br />
6<br />
3 4<br />
2<br />
26<br />
11<br />
32<br />
15<br />
9<br />
7<br />
24<br />
23<br />
16<br />
17<br />
12<br />
13<br />
25<br />
21 8<br />
14<br />
Find your nearest PBC referral<br />
hub at www.uk-pbc.com/<br />
newtherapiesinpbc/<br />
5<br />
31<br />
28<br />
29<br />
18<br />
22<br />
10<br />
20<br />
27 19<br />
Liver Centres<br />
PBC Hubs<br />
30<br />
1. Aberdeen Royal Infirmary<br />
2. Ninewells Hospital, Dundee<br />
3. Glasgow Royal Infirmary<br />
4. Edinburgh Royal Infirmary<br />
5. University Hospital Wales,<br />
Cardiff<br />
6. Royal Victoria Hospital,<br />
Belfast<br />
7. Aintree Univ Hosp NHS FT<br />
8. Barts Health NHS Trust<br />
9. Bradford Teaching<br />
10. Cambridge Univ Hosp<br />
NHS FT<br />
11. East Lancashire Hosps<br />
NHS Trust<br />
12. Hull & East Yorkshire<br />
NHS Trust<br />
13. King’s College Hosp<br />
NHS FT<br />
14. Imperial College<br />
Healthcare Trust<br />
15. Leeds Teaching Hosp<br />
NHS Trust<br />
16. Manchester University<br />
NHS FT<br />
17. Nottingham Univ Hosp<br />
NHS Trust<br />
18. Oxford Univ Hosps<br />
NHS FT<br />
19. Portsmouth Hosps<br />
NHS Trust<br />
20. Royal Devon & Exeter FT<br />
21. Royal Free London NHS FT<br />
22. Royal Surrey County Hosp<br />
NHS FT<br />
23. Royal L’pool/Broadgreen<br />
Univ Trust<br />
24. Sheffield Teaching<br />
Hosp FT<br />
25. St George’s Univ Hosps<br />
NHS FT<br />
26. The Newcastle upon Tyne<br />
Hosps FT<br />
27. Univ Hosp Southampton<br />
NHS FT<br />
28. Univ Hosps Birmingham<br />
NHS FT<br />
29. Univ Hosps Leicester<br />
NHS Trust<br />
30. Univ Hosps Plymouth<br />
NHS Trust<br />
31. University Hosp Bristol<br />
NHS FT<br />
32. York Teaching Hospital<br />
NHS FT<br />
Find out more information in the BSG guidelines at www.bsg.org.uk/clinical/bsg-guidelines.html<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
Abbreviated Prescribing Information<br />
OCALIVA ® (obeticholic acid)<br />
(Please refer to the Full Summary of Product Characteristics (SmPC) before prescribing)<br />
Presentation: OCALIVA supplied as film-coated tablets containing 5 mg<br />
and 10 mg obeticholic acid. Indication: For the treatment of primary<br />
biliary cholangitis (also known as primary biliary cirrhosis) in combination<br />
with ursodeoxycholic acid (UDCA) in adults with an inadequate response<br />
to UDCA or as monotherapy in adults unable to tolerate UDCA. Dosage<br />
and administration: Oral administration. Hepatic status must be known<br />
before initiating treatment. In patients with normal or mildly impaired<br />
(Child Pugh Class A) hepatic function, the starting dose is 5 mg once<br />
daily. Based on an assessment of tolerability after 6 months, the dose<br />
should be increased to 10 mg once daily if adequate reduction of alkaline<br />
phosphatase (ALP) and/or total bilirubin have not been achieved. No<br />
dose adjustment of concomitant UDCA is required in patients receiving<br />
obeticholic acid. For cases of severe pruritus, dose management includes<br />
reduction, temporal interruption or discontinuation for persistent<br />
intolerable pruritus; use of bile acid binding agents or antihistamines<br />
(see SmPC). Moderate to Severe Hepatic Impairment: In patients with<br />
Child-Pugh B or C hepatic impairment, a reduced starting dose of 5mg<br />
once weekly is required. After 3 months, depending on response and<br />
tolerability, the starting dose may be titrated to 5 mg twice weekly and<br />
subsequently to 10 mg twice weekly (at least 3 days between doses)<br />
if adequate reductions in ALP and/or total bilirubin have not been<br />
achieved. No dose adjustment required in Child Pugh Class A function.<br />
Mild or moderate renal impairment: No dose adjustments are required.<br />
Paediatric population: No data. Elderly: No dose adjustment required;<br />
limited data exists. Contraindications: Hypersensitivity to the active<br />
substance or any excipients. Complete biliary obstruction. Special<br />
warnings and precautions for use: After initiation, patients should be<br />
monitored for progression of PBC with frequent clinical and laboratory<br />
assessment of those at increased risk of hepatic decompensation. Dose<br />
frequency should be reduced in patients who progress from Child Pugh<br />
A to Child Pugh B or C Class disease. Serious liver injury and death have<br />
been reported in patients with moderate/severe impairment who did not<br />
receive appropriate dose reduction. Liver-related adverse events have<br />
been observed within the first month of treatment and have included<br />
elevations in alanine amino transferase (ALT), aspartate aminotransferase<br />
(AST) and hepatic decompensation. Interactions: Following coadministration<br />
of warfarin and obeticholic acid, International Normalised<br />
Ratio (INR) should be monitored and the dose of warfarin adjusted, if<br />
needed, to maintain the target INR range. Therapeutic monitoring of<br />
CYP1A2 substrates with narrow therapeutic index (e.g. theophylline and<br />
tizanidine) is recommended. Obeticholic acid should be taken at least<br />
4-6 hours before or after taking a bile acid binding resin, or at as great<br />
an interval as possible. Fertility, pregnancy and lactation: Avoid use in<br />
pregnancy. Either discontinue breast-feeding or discontinue/abstain from<br />
obeticholic acid therapy taking into account the benefit of breast-feeding<br />
for the child and the benefit of therapy for the woman. No clinical data<br />
on fertility effects. Undesirable effects: Very common (≥1/10) adverse<br />
reactions were pruritus, fatigue, and abdominal pain and discomfort. The<br />
most common adverse reaction leading to discontinuation was pruritus.<br />
The majority of pruritus occurred within the first month of treatment<br />
and tended to resolve over time with continued dosing. Other commonly<br />
(≥ 1/100 to < 1/10) reported adverse reactions are, thyroid function<br />
abnormality, dizziness, palpitations, oropharyngeal pain, constipation,<br />
eczema, rash, arthralgia, peripheral oedema, and pyrexia. Please refer to<br />
the SmPC for a full list of undesirable effects. Overdose: Liver-related<br />
adverse reactions were reported with higher than recommended doses of<br />
obeticholic acid. Patients should be carefully observed, and supportive<br />
care administered, as appropriate.<br />
Legal category: POM<br />
Marketing authorisation numbers: EU/1/16/1139/001 & 002 Marketing<br />
authorisation holder: Intercept Pharma Ltd,<br />
2 Pancras Square, London, N1C 4AG, United Kingdom Package<br />
Quantities and Basic NHS cost: OCALIVA 5 mg and 10 mg £2,384.04<br />
per bottle of 30 tablets.<br />
Date of revision: 11th April 2018<br />
Adverse events should be reported. Reporting forms and<br />
information can be found at www.mhra.gov.uk/yellowcard.<br />
Adverse events should also be reported to<br />
Intercept Pharma Ltd on +44 (0)330 100 3694<br />
or email: drugsafety@interceptpharma.com<br />
18<br />
ALP, alkaline phosphatase; PBC, primary biliary cholangitis, UDCA, ursodeoxycholic acid; ULN, upper limit of normal.<br />
1. EASL guidelines. J Hepatol. 2017;67:145–172<br />
UK-PP-PB-0376 May <strong>2019</strong>
NEWS<br />
New biosimilar safety<br />
monitoring initiative in IBD<br />
The introduction of biological treatments<br />
has made a significant difference in the<br />
lives of many IBD patients. The advent of<br />
multiple biosimilars (generic medicines that<br />
are biologically similar to the originator)<br />
increases the affordability and so availability<br />
of these life-changing treatments.<br />
How can sites participate?<br />
We are starting our monitoring of biosimilars<br />
with Hyrimoz (adalimumab), with Zessly<br />
(infliximab) coming soon.<br />
If your IBD service is using one of these<br />
biosimilars, please get in touch with us if you<br />
would like to participate in these important<br />
studies. The wider the data collection, the<br />
more closely it reflects real-world effects,<br />
so we encourage you to join with us to gather<br />
the evidence.<br />
We will be initiating a number of similar studies<br />
shortly.<br />
For further information or to get involved<br />
please contact the IBD Registry email:<br />
support@ibdregistry.org.uk<br />
Tel 020 3393 3969<br />
A primary aim of the IBD Registry is to help<br />
improve the quality of life for people with IBD,<br />
by using our UK-wide reach to collect and<br />
analyse relevant data. With over 54,000 patient<br />
records, the UK IBD Registry is one of the<br />
largest IBD registries in Europe.<br />
The recent introduction of biosimilar medicines<br />
has placed the Registry in a key position to<br />
facilitate the collection of crucial, on-going<br />
safety data as these treatments become more<br />
widely used.<br />
We are pleased to announce that the<br />
Registry will soon be facilitating a series of<br />
observational post market authorisation safety<br />
studies using our existing web-based data<br />
collection systems. Our first study will monitor<br />
the use of Hyrimoz, an adalimumab biosimilar,<br />
produced by Sandoz. The study is part of<br />
routine clinical care, but patients will be asked<br />
to consent to their data being used in the<br />
study.<br />
GI COGNITION<br />
THE MOBILE GI PHYSIOLOGY<br />
TEL: 0780 8562476<br />
INFO@GI-COGNITION.COM<br />
ANY HOSPITAL, ANYWHERE IN THE UK<br />
MOBILE DIAGNOSTIC BENEFITS<br />
• Dramatic reduction in waiting list.<br />
• Available in ALL areas of the UK.<br />
• Competitively priced, instantly cut in spending.<br />
• High standard of service and accurate reporting.<br />
• Less travelling for patients.<br />
WHO WILL BENEFIT?<br />
• Patients<br />
• Gastroenterologists<br />
• General Surgeons<br />
• Chest Specialists<br />
• ENT Specialists<br />
• GI Physiology Units<br />
• ….. Patients<br />
HOW DOES IT WORK?<br />
• Hospitals book their patients in<br />
their facility.<br />
• GI Cognition take equipment,<br />
do the tests, report.<br />
Regulated By CQC and Registered With ICO<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
19
NEWS<br />
National charity Coeliac UK<br />
and Innovate UK announce<br />
£750k boost to research that<br />
unlocks gluten free challenges<br />
Coeliac UK, the national charity for<br />
people who need to live gluten free, has<br />
combined forces with Innovate UK, the UK’s<br />
innovation agency, to drive improvements<br />
worth £750,000 in the food technology,<br />
diagnostics and digital care industries.<br />
The funding will support projects based in<br />
Birmingham, Newcastle and Edinburgh and is<br />
part of Innovate UK’s partnership with the third<br />
sector on health research projects, bringing direct<br />
benefits to both patients and UK businesses.<br />
The UK has been at the forefront of the most<br />
dynamic growth area in free from food retailing<br />
and Coeliac UK is the world’s largest support<br />
organisation for people with coeliac disease.<br />
This collaboration will build on these strengths by<br />
Sarah Sleet, chief executive of Coeliac UK<br />
said: “Coeliac UK is a world leader on coeliac<br />
disease, supporting research that makes a<br />
real world impact. This new research to create<br />
a different diagnostic test could help unlock<br />
a worldwide problem for millions of people<br />
without a proper diagnosis of coeliac disease,<br />
while the research on innovative gluten free<br />
ingredients will keep the UK ahead in the food<br />
industry’s expansion into gluten free. Meanwhile<br />
our third funded project could offer real savings<br />
to the NHS in the management of the lifelong<br />
autoimmune condition that is coeliac disease<br />
providing a service model for the many other<br />
chronic long term conditions in the UK.”<br />
Dr Kath Mackay, Director of Ageing Society,<br />
Health and Nutrition at Innovate UK, said:<br />
“Stimulating innovation in our food and<br />
health sectors are crucial components of the<br />
government’s industrial strategy. By working<br />
with Coeliac UK we will be able to offer funding<br />
that results in improved quality of life for<br />
people with this condition and support and<br />
stimulate our vibrant health care and food<br />
technology sectors.”<br />
supporting research advances in food technology,<br />
Gastro<strong>Today</strong>_Jan_<strong>2019</strong>_v4 26/01/<strong>2019</strong> 09:39 Page 1<br />
diagnostic techniques and digital care.<br />
The three projects reflect the key challenges<br />
of living with coeliac<br />
disease and a gluten<br />
free life:<br />
New test to provide<br />
a less invasive<br />
way of diagnosing<br />
coeliac disease<br />
- Nonacus Ltd,<br />
Birmingham<br />
new test will rely on a proprietary laboratory<br />
test in conjunction with a patented computer<br />
algorithm. It’s a completely new way of looking<br />
at the immune cells and can identify patients<br />
with coeliac disease by predicting how likely<br />
immune cells are to be responding to gluten.<br />
It aims to develop a coeliac disease test for<br />
people who have already adopted a gluten<br />
free diet, as well as an improvement on the<br />
current method of analysing biopsy samples.<br />
This will not only save considerable patient<br />
suffering but will also provide savings to the<br />
NHS speeding up diagnosis journeys.<br />
Development of three new plant proteins to<br />
help improve the ingredients used in gluten<br />
free bread - Nandi Proteins Ltd, Scotland<br />
To improve gluten free bread by developing<br />
revolutionary new ingredients. Nandi Proteins<br />
Ltd (a protein technology company), Genius<br />
Foods (gluten free food manufacturer) AB Mauri<br />
(distributor of bakery ingredients) and Agrii (a<br />
plant science and technology company) will join<br />
researchers at Heriot Watt University to develop<br />
three kinds of new plant proteins. The proteins<br />
will be derived from crops which are underused<br />
in the UK: rapeseed cake, faba beans and<br />
naked oats. These new ingredients could<br />
replace the expensive egg and dairy based<br />
ingredients currently used, improve the nutrient<br />
profile, taste and texture of gluten free bread<br />
and reduce the need for E number additives.<br />
Development of these new ingredients will<br />
also open up new markets for UK grown crops<br />
and add value to the UK economy. Overall<br />
consumers could see cheaper and better<br />
quality gluten free products.<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
The average time<br />
to gain a coeliac<br />
disease diagnosis is<br />
13 years and there<br />
are half a million<br />
people in the UK<br />
undiagnosed – and<br />
in the tens of millions<br />
worldwide. Nonacus<br />
Ltd, working with<br />
researchers at<br />
the University of<br />
Cambridge led by Dr<br />
Elizabeth Soilleux,<br />
will together develop<br />
a test for coeliac<br />
disease. Current<br />
tests only work if<br />
patients are still<br />
eating gluten. The<br />
Software innovation to help in the ongoing<br />
management of coeliac disease - Cievert<br />
Ltd, Newcastle<br />
Software will be developed to better manage<br />
coeliac disease. Working with leading<br />
researchers from Sheffield University, the goal<br />
is to find patients with coeliac disease that need<br />
more support, compared to those who are living<br />
well. The software, when developed, will let<br />
people receive the assurance of being clinically<br />
followed up without the inconvenience, time and<br />
cost of hospital appointments. Whilst those who<br />
need additional care will be identified quickly<br />
and easily so that they can access crucial<br />
support when they need it most. This could be<br />
technology that is applied to other conditions<br />
in the future resulting in substantial savings for<br />
the NHS.<br />
20
IMPROVING QUALITY IN<br />
NEWS<br />
DIAGNOSTIC ENDOSCOPY<br />
WITH 18 WEEK SUPPORT<br />
th<br />
Standards in colonoscopy have improved<br />
greatly over the last decade although there<br />
is still variability in the adenoma detection<br />
rates (ADR) and cancer miss rates across<br />
the UK. The bowel cancer screening program<br />
has also helped to improve standards with<br />
the introduction of competency assessment.<br />
Upper GI endoscopy however has fallen<br />
behind and cancer miss rates in the upper<br />
gastrointestinal tract remain far too high<br />
reaching between 10% and over 20%.<br />
How can we continue to improve diagnostic<br />
performance?<br />
The Endoscopy Quality Improvement Program<br />
(EQIP) for colonoscopy and endoscopy<br />
is being rolled out across the UK and has<br />
had excellent feedback from delegates.<br />
Furthermore, there is evolving evidence that<br />
training bundles are effective in improving<br />
ADR, in particular the use of position change,<br />
cleansing agents, muscular relaxants<br />
and extended withdrawal. There are other<br />
techniques which improve polyp detection<br />
including the Endocuff Vision and water<br />
exchange colonoscopy. At 18 Weeks our<br />
nurses independently measure withdrawal<br />
times and routinely polyp spot to try and<br />
improve the ADR.<br />
about Endoscopy?<br />
18 Week Support<br />
nce<br />
has<br />
00<br />
will<br />
n<br />
rces<br />
The technical aspects of upper GI endoscopy<br />
that are likely to improve the detection and<br />
diagnostic accuracy of pathology include<br />
the use of cleansing agents, in particular<br />
simethicone and N-acetyl cysteine as a<br />
mucolytic. Buscopan can aid in gastric<br />
Endoscopy views, as can services adequate gastric insufflation<br />
Our as small specialist cancers Endoscopy can hide insourcing behind gastric<br />
services folds. In have addition, been there developed is evidence to support to support<br />
NHS endoscopic trusts with: inspection time for example the<br />
Barretts 2WW Urgent inspection referrals time (BIT) taking 1 minute<br />
to Routine view each referrals 1cm of Barretts, as well as 7<br />
minutes Surveillance for the cases pre-cancerous stomach.<br />
Bowel cancer screening services<br />
The Enhanced above mentioned sedation (Propofol) techniques lists allow<br />
enhanced mucosal visualisation, however the<br />
Additionally, endoscopist we also can needs support the Direct skills to Access detect<br />
and Rapid characterise Access endoscopy mucosal pathology. referrals by The<br />
working eyes only with sees the what local the clinical mind leads is prepared to agree to<br />
strong comprehend, governance and for thus the pattern management recognition of<br />
these of pathology patients. is the key to detection and<br />
characterisation. There have been a number<br />
Criteria of recent & studies Qualitydemonstrating how detection<br />
We and select characterisation Endoscopists skills with can endoscopy be improved<br />
orientated career path and performance<br />
measures above the national average. JAG<br />
audit data is constantly monitored to ensure<br />
through image and videos based training<br />
modules. These modules can easily be<br />
viewed remotely and have the potential to<br />
improve performance both for upper and lower<br />
endscopy.<br />
18 Weeks is planning to roll out video and<br />
image training modules over the next year<br />
for 18 Weeks nurses and consultants which<br />
will be opened up to all UK based staff. Our<br />
aim is to improve endoscopic detection and<br />
characterisation.<br />
Endoscopy services<br />
Our specialist Endoscopy insourcing services<br />
have been developed to support NHS trusts<br />
with:<br />
• 2WW Urgent referrals<br />
• Routine referrals<br />
• Surveillance cases<br />
• Bowel cancer screening services<br />
• Enhanced sedation (Propofol) lists<br />
Raising the standard of care<br />
Our clinical teams are committed to<br />
Criteria & Quality<br />
working weekends to provide a wide range<br />
We select Endoscopists with an endoscopy<br />
of clinical services. Many of our clinical leads<br />
orientated career path and performance<br />
are experts in their field. So not only do<br />
measures above the national average. JAG<br />
patients benefit from the work our teams do,<br />
audit data is constantly monitored to ensure<br />
there is also an opportunity for NHS Trust<br />
ongoing quality. Furthermore, we have a<br />
teams to improve efficiencies through new<br />
clinical governance department that is crucial<br />
techniques or approaches.<br />
How our insourcing<br />
service works<br />
18 Week Support<br />
Clinical team<br />
Weekend<br />
NHS Facility NHS Staff NHS<br />
processes<br />
Happy patient<br />
Who we’re looking for<br />
We are interested in meeting with Consultant<br />
to maintaining quality and safety but also<br />
provides support to both Endoscopists and<br />
the units within which we work.<br />
Raising the standard of care<br />
Our clinical teams are committed to working<br />
weekends to provide a wide range of clinical<br />
services. Many of our clinical leads are experts<br />
in their field. So not only do patients benefit<br />
from the work our teams do, there is also an<br />
opportunity for NHS Trust teams to improve<br />
efficiencies through new techniques or<br />
approaches.<br />
An ethical company<br />
We’re an ethical and transparent company<br />
that’s s financially accountable and financially<br />
responsible. We’re committed to the NHS and<br />
the delivery of high-quality care, and to helping<br />
Trusts reduce RTT waiting times.<br />
Who we’re looking for<br />
We are interested in meeting with Consultant<br />
Gastroenterologists, senior nurses and clinical<br />
nurse specialists throughout the UK.<br />
Our remuneration package is second to none<br />
and is per session rather than per case which<br />
allows our teams to work in a safe and calm<br />
environment’<br />
About you<br />
If you have an excellent NHS record and<br />
want to help clear NHS waiting list backlogs,<br />
reduce RTT waiting times and provide<br />
high-quality patient care, get in touch by<br />
calling on 020 3892 6162 or email Gastro.<br />
Recruitment@18weeksupport.com<br />
18 Week Support<br />
www.18weeksupport.com<br />
A CQC Registered provider offering high<br />
quality clinical support services to NHS Trusts<br />
throughout the UK.<br />
London<br />
3rd Floor, 19-21 Great Tower Street London<br />
EC3R 5AR<br />
020 3869 8790<br />
Edinburgh<br />
9-10 St. Andrew Square Edinburgh EH2 2AF<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
21
NEWS<br />
Is your IBS actually<br />
undiagnosed coeliac<br />
disease?<br />
• 97%¹ of people don’t realise IBS symptoms<br />
could be coeliac disease<br />
• 1 in 4 people with coeliac disease were<br />
previously misdiagnosed with IBS<br />
• Half a million people in the UK are living with<br />
coeliac disease without knowing it<br />
With only 3% 1 of British adults aware that the<br />
symptoms of IBS (irritable bowel syndrome)<br />
are also common symptoms of coeliac<br />
disease, national charity Coeliac UK, is<br />
calling on greater awareness of the similarity<br />
of symptoms and urges anyone with IBS to<br />
ask their GP for a coeliac disease blood test,<br />
if they have not already had one.<br />
policy, research and campaigns said: “It is<br />
essential that awareness of the similarity of the<br />
symptoms increases and that GPs adhere to<br />
the NICE (National Institute for Health and Care<br />
Excellence) guideline which states that anyone<br />
with IBS symptoms should be tested for coeliac<br />
disease before a diagnosis of IBS is made.”<br />
Coeliac disease is not an allergy or an<br />
intolerance but an autoimmune disease where<br />
the body’s immune system damages the lining<br />
of the small bowel when gluten, a protein<br />
(found in wheat, barley and rye) is eaten.<br />
There is no cure and no medication; the only<br />
treatment is a strict gluten free diet for life.<br />
1 in 100 people in the UK is estimated to<br />
have coeliac disease but of these, only 30%<br />
are currently diagnosed, meaning there are<br />
nearly half a million people in the UK with<br />
undiagnosed coeliac disease.<br />
gluten ataxia and neuropathy, and although<br />
rare, there is an increased risk of small bowel<br />
cancer and intestinal lymphoma.<br />
“The first step to diagnosing coeliac disease is<br />
a simple, inexpensive blood test done in primary<br />
care, but thousands of people are not getting the<br />
necessary testing and are being left undiagnosed<br />
including those with IBS symptoms. This not<br />
only causes years of unnecessary suffering but<br />
also wasted costs to the NHS with repeated<br />
appointments and investigations.<br />
We urge anyone who has symptoms such as<br />
ongoing bloating, diarrhoea or constipation<br />
and has been given a diagnosis of IBS but not<br />
been tested for coeliac disease to ask their GP<br />
to test them for coeliac disease. However, it is<br />
essential to keep eating gluten until all tests are<br />
completed as otherwise these tests may give a<br />
false negative result,” continued Ms McGough.<br />
As many as 1 in 4 people with coeliac disease<br />
were previously misdiagnosed with IBS<br />
as many of the symptoms for IBS such as<br />
bloating, stomach pains or cramps, diarrhoea<br />
or constipation and feeling exhausted are the<br />
same as the symptoms of coeliac disease.<br />
Norma McGough Coeliac UK director of<br />
The average time it takes for someone to<br />
get a diagnosis is 13 years from the onset of<br />
symptoms; by which time, they may already be<br />
suffering with added complications caused by<br />
the disease. If left untreated, coeliac disease<br />
can lead to a number of serious complications,<br />
including: anaemia, osteoporosis, unexplained<br />
infertility, neurological conditions such as<br />
Coeliac UK’s online assessment www.coeliac.<br />
org.uk/isitcoeliacdisease, based on the NICE<br />
guideline NG20, gives people greater confidence<br />
to seek further medical advice from their GP.<br />
Upon completion of the assessment, the<br />
respondent will receive an email with their results,<br />
which will indicate whether their symptoms are<br />
potentially linked to coeliac disease.<br />
WHY NOT WRITE FOR US?<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
<strong>Gastroenterology</strong> <strong>Today</strong> welcomes the submission of<br />
clinical papers and case reports or news that<br />
you feel will be of interest to your colleagues.<br />
Material submitted will be seen by those working within all<br />
UK gastroenterology departments and endoscopy units.<br />
All submissions should be forwarded to info@mediapublishingcompany.com<br />
If you have any queries please contact the publisher Terry Gardner via:<br />
info@mediapublishingcompany.com<br />
22<br />
1<br />
Reference YouGov Survey<br />
When answering the question: ‘What do you think are common symptoms of coeliac disease?’, only 3% of respondents answered IBS symptoms.<br />
All figures, unless otherwise stated, are from YouGov Plc. Total sample size was 8423 adults. Fieldwork was undertaken between 20th December 2018 - 2nd January <strong>2019</strong>.<br />
The survey was carried out online. The figures have been weighted and are representative of all GB adults (aged 18+).
NEWS<br />
Taking endoscopy<br />
consent has never<br />
been easier<br />
Activate your patients with EIDO Verify<br />
With our new product Verify, consent to endoscopy is made simpler<br />
and safer than ever.<br />
Activate your patients before they arrive. Make alternatives, benefits<br />
and complications as simple as A B C.<br />
Verify will help reduce consent related litigation for your Trust.<br />
Contact EIDO today on 0115 878 1000 or at info@eidohealthcare.com<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
23
NEWS<br />
suicide is higher in the first six months<br />
unaddressed can have a huge detrimental<br />
Half of patients with<br />
deadliest common cancer<br />
have unmet support needs,<br />
first ever UK survey reveals<br />
of diagnosis and particularly for patients<br />
whose cancer had spread to other organs,<br />
underlining the importance of patients<br />
receiving specialist psychological care as<br />
early as possible.<br />
impact on their quality of life. Pancreatic<br />
cancer is a complex disease that can<br />
progress devastatingly quickly, often<br />
leaving those affected with little time with<br />
their loved ones. We want to see support<br />
needs assessed for all pancreatic cancer<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
Physical and psychological support need<br />
of patients with the deadliest common<br />
cancer are not being met according<br />
to the first ever UK survey into the<br />
experiences of people with pancreatic<br />
cancer. Commissioned by leading<br />
charity Pancreatic Cancer UK, the survey<br />
revealed that half of all respondents<br />
(49 per cent) had one or more unmet<br />
support needs considered either high or<br />
moderate in severity. The findings show<br />
a clear gap in the supportive care being<br />
offered to pancreatic cancer patients –<br />
a group which the charity believes has<br />
been neglected for decades.<br />
The survey, conducted by Oxford Brookes<br />
University and The Picker Institute on behalf<br />
of the charity, recorded the care experiences<br />
and support needs of 274 people with<br />
pancreatic cancer. The majority (87 per<br />
cent) reported one or more support needs,<br />
ranging from depression, fatigue, and<br />
financial pressures, to changes to appetite.<br />
Pancreatic Cancer UK is concerned that<br />
a significant proportion of these needs<br />
are not being met. It is now calling for<br />
the Government and NHS to introduce a<br />
holistic needs assessment to ensure that<br />
patients have access to personalised care<br />
immediately after diagnosis.<br />
One survey respondent said: “I was not<br />
offered counselling though I really felt I<br />
needed it. My physical needs were very well<br />
met but my emotional needs have never<br />
been addressed. I had no idea where to<br />
go for the help I needed and had to search<br />
online for information.”<br />
Patients reported that psychological care<br />
needs were the most likely to be unmet;<br />
almost a third said fears about the future<br />
(31 per cent) or fears about the cancer<br />
spreading (30 per cent) were not being<br />
addressed. This is extremely concerning as<br />
pancreatic cancer has the lowest survival of<br />
all common cancers – less than 7 per cent<br />
of people living for 5 years - and the second<br />
highest risk of suicide after diagnosis<br />
compared to other cancers. This risk of<br />
Currently there are no established<br />
psychological interventions for people<br />
with pancreatic cancer due to a lack<br />
of evidence, and the NICE guidelines<br />
on the disease cite this as a key<br />
area for improvement. The charity is<br />
urging the National Institute for Health<br />
Research (NIHR) to prioritise and invest<br />
research funding for the development of<br />
psychological interventions for people living<br />
with and beyond pancreatic cancer.<br />
Most patients were positive about the<br />
care they received, however, the findings<br />
indicated key differences between the<br />
experiences of people who were eligible to<br />
receive surgery, the only potentially curative<br />
treatment for the disease, and those whose<br />
pancreatic cancer was inoperable. People<br />
with operable pancreatic cancer were<br />
more likely to feel that their diagnosis had<br />
definitely or to some extent been given in<br />
a sensitive way (87 per cent), compared to<br />
inoperable patients (74 per cent). Similarly,<br />
37 per cent of people with inoperable<br />
pancreatic cancer reported that they had<br />
not been given enough information at the<br />
point of diagnosis, compared to 27 per cent<br />
for people with operable disease.<br />
The extent and breadth of needs and the<br />
variations in care experienced by people<br />
with pancreatic cancer has previously<br />
gone unreported because they are not all<br />
captured by the National Cancer Patient<br />
Experience Survey (NCPES). The NCPES<br />
is distributed within six to nine months<br />
of diagnosis when many people with<br />
pancreatic cancer have already died or are<br />
too sick to respond. The low number of<br />
responses mean that pancreatic cancer is<br />
grouped with Upper Gastrointestinal (UGI)<br />
cancers. The NCPES also does not capture<br />
the experience of those living beyond their<br />
diagnosis and treatment.<br />
Anna Jewell, Director of Services at<br />
Pancreatic Cancer UK, said: “For so<br />
many pancreatic cancer patients to tell us<br />
they have unmet support needs is heartbreaking<br />
- these are live needs which if left<br />
patients immediately after diagnosis so that<br />
they can be helped to maintain as good a<br />
quality of life as possible.<br />
No one affected by pancreatic cancer should<br />
be left to struggle in isolation. Specialist<br />
support is available through the Pancreatic<br />
Cancer UK Support Line. Our dedicated<br />
team of nurses are there to help patients<br />
and their families but we need fellow health<br />
professionals to signpost them to us.<br />
The needs of pancreatic cancer patients<br />
have been neglected for far too long. It’s<br />
imperative that these findings now prompt<br />
further research into the most effective<br />
interventions, particularly around mental<br />
health, so that people with pancreatic cancer<br />
receive the very best care and support.”<br />
Amy Tallett, Head of Research at Picker,<br />
commented: “Picker is proud to have<br />
worked with Pancreatic Cancer UK and<br />
Oxford Brookes University on this important<br />
research, and we hope that the findings<br />
provide an essential evidence base to<br />
inform continued conversation and actions<br />
to improve care experiences for people<br />
affected by pancreatic cancer. Thank you to<br />
everybody that took part in this research.”<br />
Eila Watson, Professor Supportive Cancer<br />
Care at Oxford Brookes University,<br />
said: “This survey highlights the unmet<br />
information and support needs that<br />
pancreatic cancer patients have across<br />
the cancer trajectory. Needs should be<br />
assessed from the point of diagnosis and<br />
monitored regularly, with supportive care<br />
interventions implemented to help patients<br />
live as good a quality of life as possible.<br />
Further research is needed to work out how<br />
best to support patients and their families.”<br />
Pancreatic Cancer UK operates the only<br />
dedicated support line for people affected by<br />
pancreatic cancer staffed by specialist nurses.<br />
The Pancreatic Cancer UK Support Line is<br />
free to call on 0808 801 0707 with support<br />
available on weekdays 10am-4pm and via<br />
email: nurse@pancreaticcancer.org.uk<br />
24
The only licensed treatment for the<br />
reduction in recurrence of overt<br />
hepatic encephalopathy (OHE) 1<br />
NEWS<br />
At home they<br />
are still at risk;<br />
…TARGAXAN ®<br />
rifaximin-α<br />
reduces the risk<br />
of recurrence<br />
of overt hepatic<br />
encephalopathy. 2<br />
Long-term secondary prophylaxis in hepatic<br />
encephalopathy (HE) 3<br />
UK&IE Prescribing Information: Targaxan 550mg (rifaximin-α)<br />
REFER TO FULL SUMMARY OF PRODUCT CHARACTERISTICS (SmPC)<br />
BEFORE PRESCRIBING<br />
Presentation: Film-coated tablet containing rifaximin 550 mg.<br />
Uses: Targaxan is indicated for the reduction in recurrence of episodes<br />
of overt hepatic encephalopathy in patients ≥ 18 years of age.<br />
Dosage and administration: Adults 18 years of age and over: 550 mg<br />
twice daily, with a glass of water, with or without food for up to 6 months.<br />
Treatment beyond 6 months should be based on risk benefit balance<br />
including those associated with the progression of the patients hepatic<br />
dysfunction. No dosage changes are necessary in the elderly or those<br />
with hepatic insufficiency. Use with caution in patients with renal<br />
impairment.<br />
Contraindications: Contraindicated in hypersensitivity to rifaximin,<br />
rifamycin-derivatives or to any of the excipients and in cases of<br />
intestinal obstruction.<br />
Warnings and precautions for use: The potential association of<br />
rifaximin treatment with Clostridium difficile associated diarrhoea and<br />
pseudomembranous colitis cannot be ruled out. The administration<br />
of rifaximin with other rifamycins is not recommended. Rifaximin<br />
may cause a reddish discolouration of the urine. Use with caution<br />
in patients with severe (Child-Pugh C) hepatic impairment and in<br />
patients with MELD (Model for End-Stage Liver Disease) score > 25.<br />
In hepatic impaired patients, rifaximin may decrease the exposure of<br />
concomitantly administered CYP3A4 substrates (e.g. warfarin,<br />
antiepileptics, antiarrhythmics, oral contraceptives). Both decreases and<br />
increases in international normalized ratio (in some cases with bleeding<br />
events) have been reported in patients maintained on warfarin and<br />
prescribed rifaximin. If co-administration is necessary, the international<br />
normalized ratio should be carefully monitored with the addition or<br />
withdrawal of treatment with rifaximin. Adjustments in the dose of<br />
oral anticoagulants may be necessary to maintain the desired level of<br />
anticoagulation. Ciclosporin may increase the rifaximin Cmax<br />
Pregnancy and lactation: Rifaximin is not recommended during<br />
pregnancy. The benefits of rifaximin treatment should be assessed<br />
against the need to continue breastfeeding.<br />
Side effects: Common effects reported in clinical trials are dizziness,<br />
headache, depression, dyspnoea, upper abdominal pain, abdominal<br />
distension, diarrhoea, nausea, vomiting, ascites, rashes, pruritus, muscle<br />
spasms, arthralgia and peripheral oedema. Other effects that have<br />
been reported include: Clostridial infections, urinary tract infections,<br />
candidiasis, pneumonia cellulitis, upper respiratory tract infection and<br />
rhinitis. Blood disorders (e.g. anaemia, thrombocytopenia). Anaphylactic<br />
reactions, angioedemas, hypersensitivity. Anorexia, hyperkalaemia and<br />
dehydration. Confusion, sleep disorders, balance disorders, convulsions,<br />
hypoesthesia, memory impairment and attention disorders.<br />
Hypotension, hypertension and fainting. Hot flushes. Breathing difficulty,<br />
pleural effusion, COPD. Gastrointestinal disorders and skin reactions.<br />
Liver function test abnormalities. Dysuria, pollakiuria and proteinuria.<br />
Oedema. Pyrexia. INR abnormalities. Prescribers should consult the<br />
SmPC in relation to all adverse reactions.<br />
UNITED KINGDOM<br />
Legal category: POM<br />
Cost: Basic NHS price £259.23 for 56 tablets<br />
Marketing Authorisation holder: Norgine Pharmaceuticals Limited,<br />
Norgine House, Widewater Place, Moorhall Road, Harefield, Uxbridge,<br />
UB9 6NS, UK<br />
Marketing Authorisation number: PL 20011/0020<br />
IRELAND<br />
Legal category: Prescription only<br />
Cost: €262.41 for 56 tablets<br />
Marketing Authorisation holder: Norgine B.V. Hogehilweg 7, 1101 CA<br />
Amsterdam ZO, Netherlands<br />
Marketing Authorisation number: PA 1336/009/001<br />
For further information contact:<br />
Norgine Pharmaceuticals Limited, Norgine House, Moorhall Road,<br />
Harefield, Middlesex UB9 6NS<br />
Telephone: 01895 826 606<br />
E-mail: Medinfo@norgine.com<br />
Ref: UK/XIF5/0119/0477<br />
Date of preparation: January <strong>2019</strong><br />
United Kingdom<br />
Adverse events should be reported. Reporting forms and<br />
information can be found at www.mhra.gov.uk/yellowcard.<br />
Adverse events should also be reported to Medical<br />
Information at Norgine Pharmaceuticals Ltd on:<br />
Tel. +44 (0)1895 826 606<br />
Email Medinfo@norgine.com<br />
Ireland<br />
Healthcare professionals are asked to report any<br />
suspected adverse reactions via HPRA Pharmacovigilance,<br />
Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971;<br />
Fax: +353 1 6762517. Website: www.hpra.ie;<br />
E-mail: medsafety@hpra.ie. Adverse events should<br />
also be reported to Medical Information at<br />
Norgine Pharmaceuticals Ltd on: Tel. +44 (0)1895 826 606<br />
Email Medinfo@norgine.com<br />
References:<br />
1. National Institute for Health and Care Excellence.<br />
Rifaximin for preventing episodes of overt hepatic encephalopathy:<br />
appraisal guidance TA337 for rifaximin.<br />
Available from: http://www.nice.org.uk/guidance/ta337<br />
2. TARGAXAN ® 550 Summary of Product Characteristics.<br />
Available for the UK from: https://www.medicines.org.uk/emc<br />
Available for Ireland from: www.medicines.ie<br />
3. Mullen KD, et al. Clin Gastroenterol Hepatol<br />
2014;12(8):1390-97.<br />
Product under licence from Alfasigma S.p.A. TARGAXAN is a<br />
registered trademark of the Alfasigma group of companies, licensed<br />
to the Norgine group of companies. NORGINE and the sail logo are<br />
registered trademarks of the Norgine group of companies.<br />
UK/XIF5/0219/0487<br />
Date of preparation: February <strong>2019</strong>.<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
25
COMPANY NEWS<br />
THE INFECTION RISK IS REAL<br />
In 2010, Cantel (UK) Ltd became the first company to offer<br />
sterile single-use endoscope valves and in 2018 became the first<br />
supplier in the UK to provide sterile single-use valves compatible<br />
with GI endoscopes from Olympus, Pentax and Fujifilm.<br />
Reducing the risk of infections for endoscopy patients is critically<br />
important. More Healthcare Associated Infections (HAIs) and outbreaks<br />
have been linked to contaminated endoscopes than to any other<br />
medical device. 1 For patients who contract HAIs the consequences<br />
can be significant. In the UK, HAIs are estimated to cost the NHS<br />
approximately £1 billion a year. 2<br />
The risk of infection from improperly cleaned and disinfected reusable<br />
endoscope valves is extremely high due to their complex design. A<br />
laboratory study of “patient-ready” valves found 56% were contaminated<br />
with bacteria, yeast, moulds and bacterial spores. 3 Meticulous brushing<br />
is required during reprocessing and that still may not be sufficient to<br />
ensure a safe, patient-ready endoscope. DEFENDO Sterile Singleuse<br />
Valves solve the issue of reusable valve reprocessing by offering a<br />
single-use option which ensures sterile valves for every procedure.<br />
DEFENDO Valves are high-performance, high-quality products that<br />
support procedural control and efficiency. Cantel’s verification testing<br />
includes multiple tests for force and suction to help create valves that<br />
don’t exhibit some of the common issues with reusable and other<br />
single-use valves: clogging, sticking and loss of insufflation. When you<br />
experience these issues during a procedure, the result can be a longer,<br />
more difficult procedure.<br />
With every guideline update, there is clear direction that singleuse<br />
accessories are highly recommended. The British Society of<br />
<strong>Gastroenterology</strong> guidance states “’Single-use’ accessories should<br />
always be used” 4 and the recently updated ESGENA guidelines state<br />
that “endoscope valves can also show contamination after reprocessing<br />
and may be the source of infections if cleaning, drying, storage, and<br />
hand hygiene are inadequate. There is an increasing trend for using<br />
detachable endoscope components as single-use products to enable<br />
full traceability and to prevent cross-infection caused by inadequately<br />
reprocessed detachable components such as valves and distal caps”. 5<br />
DEFENDO Valves provide the most advanced protection for your<br />
patients by helping to create consistent practices and reducing the risk<br />
of potential errors. Single-use valves are supplied by Cantel (UK) Ltd<br />
in a number of convenient kits which include: Air/ Water, Suction and<br />
Biopsy Valves, as well as a Single Use Auxiliary Water Jet Connector.<br />
Contact Cantel on 01702 291878 to arrange a free demonstration of<br />
DEFENDO Sterile Single-Use Valves.<br />
www.cantelmedical.co.uk<br />
References<br />
1. Rutala, W.A., Weber, D. J., and the Healthcare Infection Control<br />
Practices Advisory Committee (2008). Guideline for Disinfection<br />
and Sterilization in Healthcare Facilities (Last update: February 15,<br />
2017). Retrieved from CDC: https://www.cdc.gov/ infectioncontrol/<br />
pdf/guidelines/disinfection-guidelines.pdf<br />
2. National costing statement: Infection prevention and control (2012,<br />
March)<br />
3. Pearce, P.J. (2011, August). A Report on the Widespread<br />
Inadequate Reprocessing of Endoscope Air/Water and Suction<br />
Valves by Healthcare Facilities. Retrieved from: http://www.<br />
medivators.com/sites/default/files/minntech/documents/<br />
Improper%20Valve%20Reprocessing%20Study_Sept%20<br />
2017_50098-1504%20Rev%20A.pdf<br />
4. BSG. “Guidance for Decontamination of Equipment for<br />
Gastrointestinal Endoscopy.” (2016, November)<br />
5. Reprocessing of flexible endoscopes and endoscopic accessories<br />
used in gastrointestinal endoscopy: Position Statement of the<br />
European Society of Gastrointestinal Endoscopy (ESGE) and<br />
European Society of <strong>Gastroenterology</strong> Nurses and Associates<br />
(ESGENA) – Update 2018<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
26
COMPANY NEWS<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
27
COMPANY NEWS<br />
IMPORTANT NEW TRIAL WITH ORAL FERACCRU ® SHOWS COMPARABLE<br />
EFFICACY TO IV IRON (FERRIC CARBOXYMALTOSE), OFFERING A REAL<br />
ALTERNATIVE TO HOSPITAL ADMINISTRATION FOR MANY PATIENTS [4]<br />
• FERACCRU ® (Ferric Maltol) met primary endpoint against Ferinject ® (IV<br />
Ferric Carboxymaltose (FCM)) and shows clear benefits to Iron Deficiency<br />
Anaemia (IDA) patients with inactive Inflammatory Bowel Disease (IBD) 4<br />
• FERACCRU ® delivered a haemoglobin (Hb) response rate at 12 weeks that<br />
was within 9% of the effect seen with a market leading IV iron treatment<br />
(IVI FCM), but without the need for hospital-based administration 4<br />
• FERACCRU ® was efficiently absorbed and well tolerated over the extended<br />
treatment period, in line with previous studies 2<br />
Shield Therapeutics plc (Shield) and Norgine B.V. (Norgine) have<br />
announced positive results from the AEGIS Head-to-Head (H2H) clinical<br />
trial, which compared FERACCRU ® (ferric maltol), a novel oral iron<br />
replacement therapy, to Ferinject ® (ferric carboxymaltose (FCM)), a<br />
market-leading intravenously delivered iron replacement therapy 4 .<br />
In the AEGIS-H2H study, ferric maltol demonstrated increases in the mean<br />
Hb levels that were comparable to IV FCM. Patients with Iron Deficiency<br />
Anaemia (IDA), whose Inflammatory Bowel Disease (IBD) is inactive now<br />
have an important alternative treatment option, which is both effective<br />
over the long term and well tolerated, therefore reducing the need for<br />
time-consuming and expensive hospital administration. Current oral iron<br />
treatments can be poorly tolerated and don’t always work 7 , which leads to<br />
many unwell patients having to receive IV iron in hospital.<br />
“The results of this study provide an important opportunity to change<br />
clinical practice to improve patients’ lives. Patients with inactive<br />
Inflammatory Bowel Disease who are unwell as a result of Iron Deficiency<br />
Anaemia have often tried a number of oral iron treatments which didn’t<br />
work or they couldn’t tolerate,” commented Dr Alastair Benbow, Chief<br />
Medical and Development Officer at Norgine. “Previously, they would<br />
have needed to go to hospital for time-consuming and expensive IV<br />
administration. Now many patients can be treated at home with an<br />
effective and well-tolerated oral iron alternative,” he added.<br />
More detailed analyses of the data, including the secondary endpoints<br />
and safety parameters, will be presented at Shield’s upcoming<br />
presentation of its preliminary results for 2018, scheduled for early<br />
April <strong>2019</strong>, whilst the full data will be submitted for peer-review and<br />
subsequent presentation by the study’s lead investigators at upcoming<br />
scientific meetings.<br />
FERACCRU ® is a novel, effective and well tolerated treatment, which<br />
is approved and marketed in the European Union for the treatment of<br />
iron deficiency (ID) in adults and in Switzerland for the treatment of iron<br />
deficiency anaemia (IDA) in adults with inflammatory bowel disease<br />
(IBD) 1-3 . A New Drug Application in the USA is being reviewed by the<br />
FDA with a PDUFA date of 27 July <strong>2019</strong>.<br />
On 19 September 2018, Norgine entered into an exclusive licence<br />
agreement with Shield Therapeutics plc for the commercialisation of the<br />
product in Europe, Australia and New Zealand.<br />
WHY NOT WRITE FOR US?<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
<strong>Gastroenterology</strong> <strong>Today</strong> welcomes the submission of<br />
clinical papers and case reports or news that<br />
you feel will be of interest to your colleagues.<br />
Material submitted will be seen by those working within all<br />
UK gastroenterology departments and endoscopy units.<br />
All submissions should be forwarded to info@mediapublishingcompany.com<br />
If you have any queries please contact the publisher Terry Gardner via:<br />
info@mediapublishingcompany.com<br />
28
COMPANY NEWS<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
29
COMPANY NEWS<br />
NORGINE NEW STUDY HIGHLIGHTS NEED TO<br />
INCREASE PUBLIC UNDERSTANDING OF IMPORTANT<br />
ROLE OF COLONOSCOPY IN PREVENTING AND<br />
DIAGNOSING GASTROINTESTINAL DISEASES,<br />
INCLUDING COLORECTAL CANCER<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
30<br />
• Those with no previous experience of colonoscopy were<br />
considerably more nervous about the procedure than those<br />
who had already undergone colonoscopy (74% vs 49%)<br />
• Current lack of public understanding around colonoscopy<br />
may be negatively impacting on number of eligible people<br />
attending their colonoscopy appointment<br />
• Less than half (45%) of those who had not had the<br />
procedure knew that a colonoscopy could prevent colorectal<br />
cancer<br />
AMSTERDAM, April 4, <strong>2019</strong> /PRNewswire/ -- NORGINE B.V. (Norgine)<br />
today published the findings of a public survey at the European Society<br />
of Gastrointestinal Endoscopy (ESGE) Days in Prague as we celebrate<br />
the 50th anniversary of the first colonoscopy. [1],[2],[3] This important study<br />
highlights the need to increase public understanding of the important<br />
role of colonoscopy in the prevention and diagnosis of gastrointestinal<br />
diseases including colorectal cancer. The survey was conducted across<br />
five major European countries and included both people who had<br />
undergone a colonoscopy and those who have no previous experience<br />
with the procedure.<br />
The study findings highlighted the misconceptions and strong negative<br />
associations about colonoscopy amongst those who have had no<br />
previous experience with the procedure. Those with no previous<br />
experience of colonoscopy were considerably more nervous about the<br />
procedure than those who had already undergone colonoscopy (74% vs<br />
49%). This may be one of the reasons why many eligible people do not<br />
attend their colonoscopy appointments each year [4] - a vital procedure<br />
for the prevention and diagnosis of gastrointestinal diseases, including<br />
colorectal cancer. The target population for colorectal cancer screening<br />
in the EU is close to 69,000,000, but only 14% of the target population is<br />
currently being screened. [4]<br />
This study has highlighted the benefit of public education to increase<br />
understanding of the importance of the colonoscopy procedure, and<br />
particularly its important role in preventing colorectal cancer. Less<br />
than half (45%) of those who had not had the procedure knew that a<br />
colonoscopy could prevent colorectal cancer. Other findings suggested<br />
the need to improve patient experience of the procedure, including<br />
bowel preparation and the provision of relevant information. This may<br />
provide an opportunity for healthcare professionals to further support<br />
their patients.<br />
“This survey highlights the lack of information about colonoscopy in<br />
public domain. Clinicians need to provide easily accessible and clear<br />
information about colonoscopy to improve the uptake of bowel cancer<br />
screening program in our fight against colorectal cancer,” said Professor<br />
Pradeep Bhandari, Consultant Gastroenterologist, QA Hospital,<br />
Portsmouth.<br />
In the 50 years since the first colonoscopy, the procedure has become a<br />
crucial tool in the prevention and detection of gastrointestinal disorders,<br />
including colorectal cancer. Despite significant advances, however, the<br />
variation in uptake across Europe continues to prevent the potential of<br />
colonoscopy being fully realised for patients and health systems.<br />
The survey asked 500 and 2500 people with and without colonoscopy<br />
experience across five main EU countries (UK, Germany, France, Spain<br />
and Italy) about their experience and understanding of colonoscopy.<br />
[1],[2],[3]<br />
Follow us @norgine<br />
www.norgine.com<br />
References<br />
[1] Bhandari P, Amlani B, Radaelli F. ePP31 Public attitudes to<br />
colonoscopy: The purpose of colonoscopy. Presented at ESGE<br />
<strong>2019</strong>, Prague. https://www.professionalabstracts.com/esge<strong>2019</strong>/<br />
iplanner/#/presentation/336 Last accessed April <strong>2019</strong><br />
[2] Amlani B, Bhandari P, Radaelli F. ePP92 Public attitudes to<br />
colonoscopy: Experience of colonoscopy. Presented at ESGE<br />
<strong>2019</strong>, Prague. https://www.professionalabstracts.com/esge<strong>2019</strong>/<br />
iplanner/#/presentation/409 Last accessed April <strong>2019</strong><br />
[3] Bhandari P, Amlani B, Radaelli F. OP362 Public attitudes to<br />
colonoscopy: Embarrassment levels and colonoscopy. Presented<br />
at ESGE <strong>2019</strong>, Prague. https://www.professionalabstracts.com/<br />
esge<strong>2019</strong>/iplanner/#/presentation/890 Last accessed April <strong>2019</strong><br />
[4] European Commission, Cancer Screening in the European Union,<br />
Report on the implementation of the Council recommendation on<br />
cancer screening, 2017. https://ec.europa.eu/health/sites/health/<br />
files/major_chronic_diseases/docs/2017_cancerscreening_2ndrepo<br />
rtimplementation_en.pdf Last accessed April <strong>2019</strong>
COMPANY NEWS<br />
driving scientific advancements in digestive health<br />
October 19-23, <strong>2019</strong><br />
Venue: Fira Gran Via, Barcelona<br />
Ahead of UEG Week Barcelona <strong>2019</strong>, UEG<br />
President Professor Paul Fockens discusses<br />
why he is looking forward to one of the<br />
world’s premier digestive health meetings.ngs.<br />
UEG Week is the largest<br />
and most prestigious<br />
gastroenterology meeting<br />
of its kind, with more than 14,000<br />
delegates from over 110 countries<br />
worldwide.<br />
The UEG Scientific Committee is<br />
developing a state-of-the-art<br />
scientific programme, featuring the<br />
latest advancements and most exciting<br />
research in digestive health.<br />
This will ensure the delivery of<br />
world-class presentations across a<br />
range of specialties, covering clinical,<br />
translational and basic science. An<br />
inclusive offering is provided for all<br />
attendees, whatever their level of<br />
expertise, featuring a broad variety<br />
of sessions that include symposia,<br />
live endoscopy and abstract-based<br />
sessions. Different interactive formats<br />
allow lively interaction between the<br />
audience, chairs and speakers.<br />
The two-day Postgraduate Teaching<br />
Programme will provide profound<br />
updates on a wide range of<br />
gastrointestinal and hepatology topics<br />
that suit both gastroenterologists<br />
in training as well as established<br />
physicians and general practitioners.<br />
The programme focuses on the<br />
relevance for the clinical day-to-day<br />
business and enables participants<br />
to get perfectly prepared for their<br />
professional career. <strong>2019</strong> marks year 3<br />
of our rolling 3-year curriculum.<br />
UEG Week will once again host the<br />
hugely successful ‘<strong>Today</strong>’s Science,<br />
Tomorrow’s Medicine’ initiative and<br />
this year’s theme will be ‘Microbiota:<br />
Moving towards clinics’. Here, topclass<br />
scientists will be invited to<br />
discuss how current knowledge and<br />
thinking is ready to be used in clinical<br />
practice and establish strategies to<br />
foster further progression in this area.<br />
UEG Week provides an exceptional<br />
opportunity for investigators from<br />
around the globe to submit and<br />
present their latest findings. UEG will<br />
present a number of awards at the<br />
congress, including the Top Abstract<br />
Prizes, the UEG Research Prize and the<br />
UEG Rising Star Awards.<br />
I am anticipating a very exciting<br />
week of scientific advances and<br />
updates from leading digestive health<br />
experts and thoroughly look forward<br />
to welcoming new and returning<br />
delegates to UEG Week Barcelona<br />
<strong>2019</strong>.<br />
To find out more,<br />
visit: ueg.eu/week<br />
Benefit from<br />
reduced delegate<br />
fees until<br />
September 5,<br />
<strong>2019</strong><br />
Late-breaking<br />
abstract<br />
submission<br />
opens August<br />
19, <strong>2019</strong><br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
31
COMPANY NEWS<br />
NEW CHIEF EXECUTIVE OFFICER ANNOUNCEMENT<br />
Coeliac UK, the national charity for people who need to live<br />
gluten free, has appointed Hilary Croft as its new CEO from<br />
3 June <strong>2019</strong>, succeeding Sarah Sleet.<br />
Ms Croft’s professional background lies in business transformation<br />
within complex, multifaceted organisations. Her senior management<br />
career started in marketing with Capgemini, a global leader in consulting,<br />
technology and digital transformation services, where she worked with a<br />
variety of organisations, such as Virgin Atlantic, Sky, Lego and Royal Mail.<br />
Hilary Croft also has vast knowledge and interest in the food and drink<br />
sector through previous positions with Marks and Spencer, Coca-Cola,<br />
Compass Group and World Duty Free Europe. More recently, as CEO<br />
of the Felix Project, she developed significant partnerships with food<br />
suppliers and re-distributers to reduce food waste and food poverty<br />
across London.<br />
About her appointment, Ms Croft said: “I am excited to be taking<br />
up the CEO role at Coeliac UK, a charity with a truly unique health<br />
and food scope. I look forward to developing the charity’s strong<br />
reputation, bringing fresh ideas and strategic insights. Naturally, my<br />
direct experience of coeliac disease, through my son’s condition, further<br />
motivates me to achieve real and lasting change for the gluten free<br />
community.”<br />
Mike Elliott, Coeliac UK’s Chair of Governors, said: “Hilary’s business<br />
and strategic acumen will bring new drive and impetus at this exciting<br />
time in Coeliac UK’s journey. The charity is currently entering a new ten<br />
year strategic review and requires an experienced change leader to<br />
adapt to a constantly evolving, complex environment. We feel Hilary is<br />
exceptionally qualified to lead Coeliac UK into the future and we look<br />
forward to welcoming her.”<br />
Ms Croft is also a trustee of Age UK Ealing and a global fundraising<br />
committee member for Tearfund, a charity that helps communities<br />
around the world escape the very worst effects of poverty and disaster.<br />
About Coeliac UK<br />
Coeliac UK campaigns for better access to diagnosis of coeliac disease<br />
and funds critical research into potential cures. It provides expert and<br />
independent information to 65,000 members to manage their health and<br />
gluten free diet.<br />
The charity also fights for wider availability of gluten free food by working<br />
with food manufacturers, service providers and venues. Currently 3,000<br />
products and 200 companies use the charity’s Crossed Grain certification<br />
scheme and 3,200 food outlets, cafés and restaurants have achieved its<br />
Gluten Free accreditation. In 2018, the charity’s total income was £4.1m<br />
with research expenditure increasing from £204k to £496k.<br />
WHY NOT WRITE FOR US?<br />
<strong>Gastroenterology</strong> <strong>Today</strong> welcomes the submission of<br />
clinical papers and case reports or news that<br />
you feel will be of interest to your colleagues.<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
Material submitted will be seen by those working within all<br />
UK gastroenterology departments and endoscopy units.<br />
All submissions should be forwarded to info@mediapublishingcompany.com<br />
If you have any queries please contact the publisher Terry Gardner via:<br />
info@mediapublishingcompany.com<br />
32
COMPANY NEWS<br />
THE GUT HEALTH EXPERTS<br />
One of the<br />
UK’s leading<br />
digestive<br />
brands *<br />
Scientifically<br />
studied<br />
strains<br />
Over<br />
30 years<br />
experience<br />
Billions<br />
of live<br />
bacteria<br />
FRIDGE<br />
FREE<br />
technology<br />
DISCOVER THE BIOGLAN DIFFERENCE<br />
It has literally changed<br />
my life. I no longer have<br />
a single symptom of<br />
Colitis and have absolute<br />
freedom with my life.<br />
Your product is amazing.<br />
- S.Firth, <strong>2019</strong><br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
*based on Euromonitor brand sales<br />
www.bioglan.co.uk<br />
33
COMPANY NEWS<br />
NORGINE: NEW REAL WORLD STUDY SHOWS<br />
RIFAXIMIN-A SIGNIFICANTLY REDUCED<br />
HOSPITALISATION IN PATIENTS WITH OVERT HEPATIC<br />
ENCEPHALOPATHY (HE) WHEN ADDED TO LACTULOSE<br />
• Rifaximin-a in combination with lactulose, the standard of<br />
care (SOC) treatment resulted in significant reductions<br />
(p
COMPANY NEWS<br />
GASTROENTEROLOGY TODAY - SUMMER <strong>2019</strong><br />
35
Helicobacter Test INFAI ®<br />
The most used 13 C-urea breath test for the<br />
diagnosis of Hp-infection worldwide<br />
• more than 4.5 million INFAI tests performed in Europe<br />
• approved for children from the ages of 3 to 11<br />
• special INFAI test for patients with dyspepsia taking PPIs<br />
• cost-effective CliniPac Basic version for hospital use<br />
INFAI Institute for Biomedical Analysis & NMR Imaging, INFAI UK Ltd<br />
Innovation Centre, University Science Park, University Road, Heslington, YORK YO10 5DG, UK<br />
Phone +44 1904 435 228 - Fax +44 1904 435 229 - mail: info@infai.co.uk - Visit us at www.infai.com