bioplasticsMAGAZINE_1205
bioplasticsMAGAZINE_1205
bioplasticsMAGAZINE_1205
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Polyurethanes / Elastomers<br />
CO 2<br />
C<br />
CH 3<br />
H 3<br />
C H 3<br />
C O<br />
O<br />
O<br />
C<br />
C<br />
C CH 2<br />
H 3<br />
C CH 2<br />
H 3<br />
C CH 2<br />
H 3<br />
C<br />
O<br />
O O<br />
Limonene<br />
C<br />
+ H 2<br />
N<br />
O<br />
O<br />
H 3<br />
C<br />
OH<br />
O<br />
C<br />
O<br />
NH<br />
Limonene<br />
dicarbonate<br />
O<br />
C<br />
C<br />
O<br />
C<br />
H 3<br />
C<br />
OH<br />
H 2<br />
bioplastics MAGAZINE [05/12] Vol. 7 41<br />
NH<br />
NIPU<br />
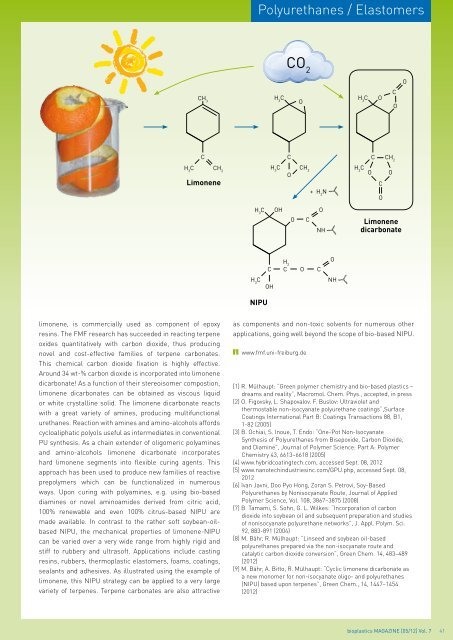
limonene, is commercially used as component of epoxy<br />
resins. The FMF research has succeeded in reacting terpene<br />
oxides quantitatively with carbon dioxide, thus producing<br />
novel and cost-effective families of terpene carbonates.<br />
This chemical carbon dioxide fixation is highly effective.<br />
Around 34 wt-% carbon dioxide is incorporated into limonene<br />
dicarbonate! As a function of their stereoisomer compostion,<br />
limonene dicarbonates can be obtained as viscous liquid<br />
or white crystalline solid. The limonene dicarbonate reacts<br />
with a great variety of amines, producing multifunctional<br />
urethanes. Reaction with amines and amino-alcohols affords<br />
cycloaliphatic polyols useful as intermediates in conventional<br />
PU synthesis. As a chain extender of oligomeric polyamines<br />
and amino-alcohols limonene dicarbonate incorporates<br />
hard limonene segments into flexible curing agents. This<br />
approach has been used to produce new families of reactive<br />
prepolymers which can be functionalized in numerous<br />
ways. Upon curing with polyamines, e.g. using bio-based<br />
diamines or novel aminoamides derived from citric acid,<br />
100% renewable and even 100% citrus-based NIPU are<br />
made available. In contrast to the rather soft soybean-oilbased<br />
NIPU, the mechanical properties of limonene-NIPU<br />
can be varied over a very wide range from highly rigid and<br />
stiff to rubbery and ultrasoft. Applications include casting<br />
resins, rubbers, thermoplastic elastomers, foams, coatings,<br />
sealants and adhesives. As illustrated using the example of<br />
limonene, this NIPU strategy can be applied to a very large<br />
variety of terpenes. Terpene carbonates are also attractive<br />
as components and non-toxic solvents for numerous other<br />
applications, going well beyond the scope of bio-based NIPU.<br />
www.fmf.uni-freiburg.de<br />
[1] R. Mülhaupt: “Green polymer chemistry and bio-based plastics –<br />
dreams and reality”, Macromol. Chem. Phys., accepted, in press<br />
[2] O. Figovsky, L. Shapovalov. F. Buslov: Ultraviolet and<br />
thermostable non-isocyanate polyurethane coatings“,Surface<br />
Coatings International Part B: Coatings Transactions 88, B1,<br />
1-82 (2005)<br />
[3] B. Ochiai, S. Inoue, T. Endo: “One-Pot Non-Isocyanate<br />
Synthesis of Polyurethanes from Bisepoxide, Carbon Dioxide,<br />
and Diamine”, Journal of Polymer Science: Part A: Polymer<br />
Chemistry 43, 6613–6618 (2005)<br />
[4] www.hybridcoatingtech.com, accessed Sept. 08, 2012<br />
[5] www.nanotechindustriesinc.com/GPU.php, accessed Sept. 08,<br />
2012<br />
[6] Ivan Javni, Doo Pyo Hong, Zoran S. Petrovi, Soy-Based<br />
Polyurethanes by Nonisocyanate Route, Journal of Applied<br />
Polymer Science, Vol. 108, 3867–3875 (2008)<br />
[7] B. Tamami, S. Sohn, G. L. Wilkes: “Incorporation of carbon<br />
dioxide into soybean oil and subsequent preparation and studies<br />
of nonisocyanate polyurethane networks”, J. Appl. Polym. Sci.<br />
92, 883-891 (2004)<br />
[8] M. Bähr, R. Mülhaupt: “Linseed and soybean oil-based<br />
polyurethanes prepared via the non-isocyanate route and<br />
catalytic carbon dioxide conversion”, Green Chem. 14, 483–489<br />
(2012)<br />
[9] M. Bähr, A. Bitto, R. Mülhaupt: “Cyclic limonene dicarbonate as<br />
a new monomer for non-isocyanate oligo- and polyurethanes<br />
(NIPU) based upon terpenes”, Green Chem., 14, 1447–1454<br />
(2012)