atw - International Journal for Nuclear Power | 05.2021
Description Ever since its first issue in 1956, the atw – International Journal for Nuclear Power has been a publisher of specialist articles, background reports, interviews and news about developments and trends from all important sectors of nuclear energy, nuclear technology and the energy industry. Internationally current and competent, the professional journal atw is a valuable source of information. www.nucmag.com
Description
Ever since its first issue in 1956, the atw – International Journal for Nuclear Power has been a publisher of specialist articles, background reports, interviews and news about developments and trends from all important sectors of nuclear energy, nuclear technology and the energy industry. Internationally current and competent, the professional journal atw is a valuable source of information.
www.nucmag.com
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>atw</strong> Vol. 66 (2021) | Issue 5 ı September<br />
RESEARCH AND INNOVATION 56<br />
against time gives a composite curve<br />
in which the activity initially decreases<br />
sharply and thereafter very<br />
slowly giving nearly straight line,<br />
evidently rapid and slow ion adsorption<br />
reactions were occurring simultaneously<br />
[19]. The activity exchanged<br />
due to rapid, slow uptake reactions as<br />
well as the specific reaction rate (k)<br />
of rapid ion uptake reactions were<br />
calculated in the same way as<br />
explained previously [20-22]. The<br />
amount of bromide ions exchanged<br />
(mmol) on the resin were obtained<br />
from the initial and final activity of<br />
solution and the amount of ions in<br />
200mL of solution.<br />
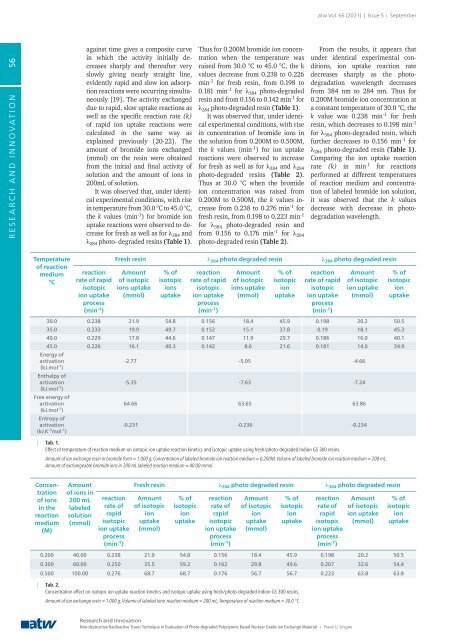
It was observed that, under identical<br />
experimental conditions, with rise<br />
in temperature from 30.0 °C to 45.0 °C,<br />
the k values (min -1 ) <strong>for</strong> bromide ion<br />
uptake reactions were observed to decrease<br />
<strong>for</strong> fresh as well as <strong>for</strong> λ 384 and<br />
λ 284 photo- degraded resins (Table 1).<br />
Thus <strong>for</strong> 0.200M bromide ion concentration<br />
when the temperature was<br />
raised from 30.0 °C to 45.0 °C, the k<br />
values decrease from 0.238 to 0.226<br />
min -1 <strong>for</strong> fresh resin, from 0.198 to<br />
0.181 min -1 <strong>for</strong> λ 384 photo-degraded<br />
resin and from 0.156 to 0.142 min -1 <strong>for</strong><br />
λ 284 photo-degraded resin (Table 1).<br />
It was observed that, under identical<br />
experimental conditions, with rise<br />
in concentration of bromide ions in<br />
the solution from 0.200M to 0.500M,<br />
the k values (min -1 ) <strong>for</strong> ion uptake<br />
reactions were observed to increase<br />
<strong>for</strong> fresh as well as <strong>for</strong> λ 384 and λ 284<br />
photo-degraded resins (Table 2).<br />
Thus at 30.0 °C when the bromide<br />
ion concentration was raised from<br />
0.200M to 0.500M, the k values increase<br />
from 0.238 to 0.276 min -1 <strong>for</strong><br />
fresh resin, from 0.198 to 0.223 min -1<br />
<strong>for</strong> λ 384 photo-degraded resin and<br />
from 0.156 to 0.176 min -1 <strong>for</strong> λ 284<br />
photo-degraded resin (Table 2).<br />
From the results, it appears that<br />
under identical experimental conditions,<br />
ion uptake reaction rate<br />
decreases sharply as the photodegradation<br />
wavelength decreases<br />
from 384 nm to 284 nm. Thus <strong>for</strong><br />
0.200M bromide ion concentration at<br />
a constant temperature of 30.0 °C, the<br />
k value was 0.238 min -1 <strong>for</strong> fresh<br />
resin, which decreases to 0.198 min -1<br />
<strong>for</strong> λ 384 photo-degraded resin, which<br />
further decreases to 0.156 min -1 <strong>for</strong><br />
λ 284 photo-degraded resin (Table 1).<br />
Comparing the ion uptake reaction<br />
rate (k) in min -1 <strong>for</strong> reactions<br />
per<strong>for</strong>med at different temperatures<br />
of reaction medium and concentration<br />
of labeled bromide ion solution,<br />
it was observed that the k values<br />
decrease with decrease in photodegradation<br />
wavelength.<br />
Temperature<br />
of reaction<br />
medium<br />
°C<br />
reaction<br />
rate of rapid<br />
isotopic<br />
ion uptake<br />
process<br />
(min -1 )<br />
Fresh resin λ 284 photo degraded resin λ 384 photo degraded resin<br />
Amount<br />
of isotopic<br />
ions uptake<br />
(mmol)<br />
% of<br />
isotopic<br />
ions<br />
uptake<br />
reaction<br />
rate of rapid<br />
isotopic<br />
ion uptake<br />
process<br />
(min -1 )<br />
Amount<br />
of isotopic<br />
ions uptake<br />
(mmol)<br />
% of<br />
isotopic<br />
ion<br />
uptake<br />
reaction<br />
rate of rapid<br />
isotopic<br />
ion uptake<br />
process<br />
(min -1 )<br />
Amount<br />
of isotopic<br />
ion uptake<br />
(mmol)<br />
% of<br />
isotopic<br />
ion<br />
uptake<br />
30.0 0.238 21.9 54.8 0.156 18.4 45.9 0.198 20.2 50.5<br />
35.0 0.233 19.9 49.7 0.152 15.1 37.8 0.19 18.1 45.3<br />
40.0 0.229 17.8 44.6 0.147 11.9 29.7 0.186 16.0 40.1<br />
45.0 0.226 16.1 40.3 0.142 8.6 21.6 0.181 14.0 34.9<br />
Energy of<br />
activation<br />
(kJ.mol -1 )<br />
Enthalpy of<br />
activation<br />
(kJ.mol -1 )<br />
Free energy of<br />
activation<br />
(kJ.mol -1 )<br />
Entropy of<br />
activation<br />
(kJ.K -1 mol -1 )<br />
-2.77 -5.05 -4.66<br />
-5.35 -7.63 -7.24<br />
64.66 63.65 63.86<br />
-0.231 -0.236 -0.234<br />
| Tab. 1.<br />
Effect of temperature of reaction medium on isotopic ion uptake reaction kinetics and isotopic uptake using fresh/photo-degraded Indion GS 300 resins.<br />
Amount of ion exchange resin in bromide <strong>for</strong>m = 1.000 g; Concentration of labeled bromide ion reaction medium = 0.200M; Volume of labeled bromide ion reaction medium = 200 mL;<br />
Amount of exchangeable bromide ions in 200 mL labeled reaction medium = 40.00 mmol.<br />
Concentration<br />
of ions<br />
in the<br />
reaction<br />
medium<br />
(M)<br />
Amount<br />
of ions in<br />
200 mL<br />
labeled<br />
solution<br />
(mmol)<br />
reaction<br />
rate of<br />
rapid<br />
isotopic<br />
ion uptake<br />
process<br />
(min -1 )<br />
Fresh resin λ 284 photo degraded resin λ 384 photo degraded resin<br />
Amount<br />
of isotopic<br />
ion<br />
uptake<br />
(mmol)<br />
% of<br />
isotopic<br />
ion<br />
uptake<br />
reaction<br />
rate of<br />
rapid<br />
isotopic<br />
ion uptake<br />
process<br />
(min -1 )<br />
Amount<br />
of isotopic<br />
ion<br />
uptake<br />
(mmol)<br />
% of<br />
isotopic<br />
ion<br />
uptake<br />
reaction<br />
rate of<br />
rapid<br />
isotopic<br />
ion uptake<br />
process<br />
(min -1 )<br />
Amount<br />
of isotopic<br />
ion uptake<br />
(mmol)<br />
% of<br />
isotopic<br />
ion<br />
uptake<br />
0.200 40.00 0.238 21.9 54.8 0.156 18.4 45.9 0.198 20.2 50.5<br />
0.300 60.00 0.250 35.5 59.2 0.162 29.8 49.6 0.207 32.6 54.4<br />
0.500 100.00 0.276 68.7 68.7 0.176 56.7 56.7 0.223 63.8 63.8<br />
| Tab. 2.<br />
Concentration effect on isotopic ion uptake reaction kinetics and isotopic uptake using fresh/photo-degraded Indion GS 300 resins.<br />
Amount of ion exchange resin = 1.000 g; Volume of labeled ionic reaction medium = 200 mL; Temperature of reaction medium = 30.0 °C.<br />
Research and Innovation<br />
Non-destructive Radioactive Tracer Technique in Evaluation of Photo- degraded Polystyrene Based <strong>Nuclear</strong> Grade Ion Exchange Material ı Pravin U. Singare