Gschwend%20thesis.pdf
Gschwend%20thesis.pdf
Gschwend%20thesis.pdf
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
102 R. P. SCHWARZENBACH, R. H. BROMUND. P. M. GSCHWEND and O. C ZAFIRIOU<br />
tadecene ()'oungblood et al.. 1971), C¡ i alkenes (dictyopterenes,<br />
Moore, 1977) and pristane (Blumer et al..<br />
1964) at CD is probably much less than that of n-C i 5<br />
and /J-C 17' Assuming the same turnover time with<br />
respect to air-sea gas exchange (Equation (2)), the<br />
resultant steady-state concentrations can be expected<br />
to be undetectably low. Additional sinks, such as<br />
adsorption and mixing with offshore waters can only<br />
lower the steady-state levels even further.<br />
Aromatic hydrocarhons<br />
Toluene and many of the isomeric C2 to C4 alkyl<br />
benzenes are present; together they form the group<br />
of compounds most abundant and consistently<br />
present at CD. They are found in all samples, despite<br />
our precautions to minimize or eliminate contamination<br />
by these compounds. They are absent in the<br />
blanks and in five liter samples of air from CD.<br />
Therefore we believe that they are not artifacts, but<br />
are actually present in the samples. Toluene (No. 45)<br />
is often a major peak; the other alkylated benzene<br />
concentrations covary. The isomer distributions are<br />
conveniently displayed as (M + 1)+ mass chromatograms<br />
of. the CI (methane) mass spectra data files.<br />
Making the reasonable assumption that these isomers<br />
show similar proton affnities, the normalized<br />
(M + i) + mass chromatograms give the isomer distributions<br />
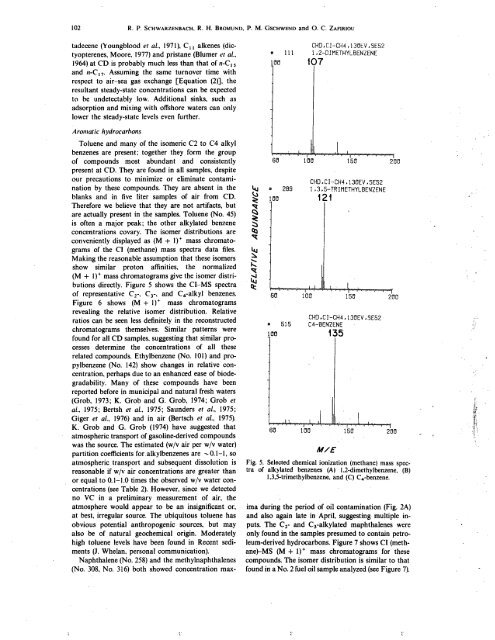
directly. Figure 5 shows the CI-MS spectra<br />
of representative Ci-, Ci-, and C4-alkyl benzenes.<br />
Figure 6 shows (M + 1)+ mass chromatograms<br />
revealing the relative isomer distribution. Relative<br />
ratios can be seen less definitely in the reconstructed<br />
chromatograms themselves. Similar patterns were<br />
found for aII CD samples, suggesting that similar pro-<br />
cesses determine the concentrations of aII these<br />
related compounds. Ethylbenzene (No. 101) and propylbenzene<br />
(No. 142) show changes in relative concentration,<br />
perhaps due to an enhanced ease of biodegradability.<br />
Many of these compounds have been<br />
reported before in municipal and natural fresh waters<br />
(Grob, 1973; K. Grob and G. Grob, 1974; Grob et<br />
al., 1975; Bertsh et aI., 1975; Saunders et al., 1975;<br />
Giger et al., 1976) and in air (Bertsch et aI., 1975).<br />
K. Grob and G. Grob (1974) have suggested that<br />
atmospheric transport of gasoline-derived compounds<br />
was the source. The estimated (wlv air per wlv water)<br />
partition coeffcients foralkylbenzenes are - 0.1- i, so<br />
atmòspheric transport and subsequent dissolution is<br />
reasonable if wlv air concentrations are greater than<br />
or equal to 0.1-1.0 times the observed wlv water concentrations<br />
(see Table 2). However, since we detected<br />
no VC in a preliminary measurement of air, the<br />
atmosphere would appear to be an insignificant or,<br />
at best, irregular source. The ubiquitous toluene has<br />
obvious potential anthropogenic sources, but may<br />
also be of natural geochemical origin. Moderately<br />
high toluene levels have been found in Recent sediments<br />
(J. Whelan, personal communication).<br />
Naphthalene (No. 258) and the methylnaphthalenes<br />
(No. 308, No. 316) both showed concentration max-<br />
~ ~<br />
""<br />
~<br />
Qi<br />
""<br />
~<br />
i:<br />
""<br />
~<br />
It ill<br />
ICJCJ<br />
CHO .Cl-CH4 ,I3CJlV .5ES2<br />
1.2-01METHYLBENZENE<br />
107<br />
6CJ ICJCJ ISCJ 2CJO<br />
It 289<br />
ICJCJ<br />
CHO .CI-CH4 .130EY .SES2<br />
I .3 .S~ TRIMETHYLBENZENE<br />
121<br />
60 10CJ ISCJ 2CJO<br />
It SI5<br />
ICJCJ<br />
CHO . C I~CH4 . 13CJEV .5E52<br />
C4-BENZENE<br />
135<br />
6CJ 100 ISO 20CJ<br />
M/£<br />
Fig. 5. Selected chemical ionization (methane) mass spectra<br />
of alkylated benzenes (A) 1,2-dimethylbenzene, (B)<br />
1,3,5-trimethylbenzene, and (C) C4-benzene.<br />
ima during the period of oil contamination (Fig. 2A)<br />
and also again late in April, suggesting multiple in-<br />
puts. The Ci- and Ci-alkylated maphthalenes were<br />
only found in the samples presumed to contain petroleum-derived<br />
hydrocarbons. Figure 7 shows CI (methane)-MS<br />
(M + 1)+ mass chromatograms for these<br />
compounds. The isomer distribution is similar to that<br />
found in a No.2 fuel oil sample analyzed (see Figure 7).<br />
i,'~<br />
':r<br />
*-<br />
r: