Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
10 J. E. R. Sadig, M. C. Willis REVIEW<br />
Me<br />
HN<br />
H2N<br />
+<br />
N<br />
Scheme 26<br />
2.4 Indazoles <strong>and</strong> Indazolones<br />
Indazoles have proven to be popular targets for amination<br />
chemistry. A number of groups have described the cyclization<br />
of appropriately substituted arylhydrazones.<br />
Scheme 27 illustrates an intramolecular coupling of bromo-substituted<br />
arylhydrazone 57 to deliver 1H-indazole<br />
58 in 85% yield. 72 The DPEPhos-derived catalyst system<br />
was effective for a wide range of substrates, although aryl<br />
chloride substrates performed poorly. Analogous N-tosylhydrazones<br />
were also established as effective indazole<br />
precursors, 73a <strong>and</strong> were utilized in a synthesis of the natural<br />
product nigellicine. 73b 3-Amino-1H-indazoles were<br />
also prepared by similar palladium-catalyzed cyclizations.<br />
74 Song <strong>and</strong> Yee demonstrated that appropriately<br />
substituted hydrazines are also useful indazole precursors.<br />
For example, palladium-catalyzed ring closure using hydrazine<br />
59 delivered the corresponding aromatic 1H-indazole<br />
directly in 87% yield, following intramolecular<br />
amination <strong>and</strong> spontaneous aromatization. 75 The mechanism<br />
of aromatization was not established. The authors<br />
noted the instability of certain hydrazine substrates to<br />
long-term storage, <strong>and</strong> as alternatives established that the<br />
corresponding N-triphenylphosphonium bromide salts<br />
provided convenient stable precursors that could be cyclized<br />
under identical reaction conditions.<br />
MeO<br />
57<br />
Scheme 27<br />
Br<br />
I<br />
Br<br />
N<br />
O<br />
CuI (15 mol%)<br />
25 (30 mol%)<br />
Cs2CO3<br />
DMA, 165 °C<br />
N<br />
N O<br />
N<br />
H<br />
53%<br />
There are a number of reports of halo-substituted hydrazones<br />
being formed in situ <strong>and</strong> then cyclized to yield 1Hindazoles;<br />
Scheme 28 presents examples using both palladium<br />
<strong>and</strong> copper catalysis. Cho et al. were able to show<br />
that o-bromobenzaldehydes could be combined with phenylhydrazine<br />
using a palladium(II) chloride/dppp catalyst<br />
system to furnish the corresponding indazoles in good<br />
yields (60 → 61). 76 The copper example, reported by<br />
Synthesis 2011, No. 1, 1–22 © Thieme Stuttgart · New York<br />
Me<br />
Me<br />
H<br />
N<br />
Pd(dba)2 (2 mol%<br />
N<br />
DPEPhos (22) (2 mol%)<br />
N<br />
Me<br />
NH<br />
K 3PO 4<br />
toluene, 110 °C<br />
MeO<br />
Pd(OAc) 2 (5 mol%)<br />
dppf (12) (7.5 mol%)<br />
Me<br />
58, 85%<br />
Br HN<br />
NaOt-Bu<br />
toluene, 90 59 °C<br />
87%<br />
N<br />
N<br />
Me<br />
Pabba et al., required a two-step one-pot approach in<br />
which a ten-minute microwave reaction was used to form<br />
the hydrazone before a copper(I)/diamine catalyst was<br />
added to the system (62 → 63). 77 Del Olmo <strong>and</strong> co-workers<br />
reported a related copper-catalyzed process which also<br />
allowed the use of aryl carboxylic acid substrates to deliver<br />
1-hydroxy-1H-indazoles. 78<br />
MeO<br />
MeO<br />
F<br />
Scheme 28<br />
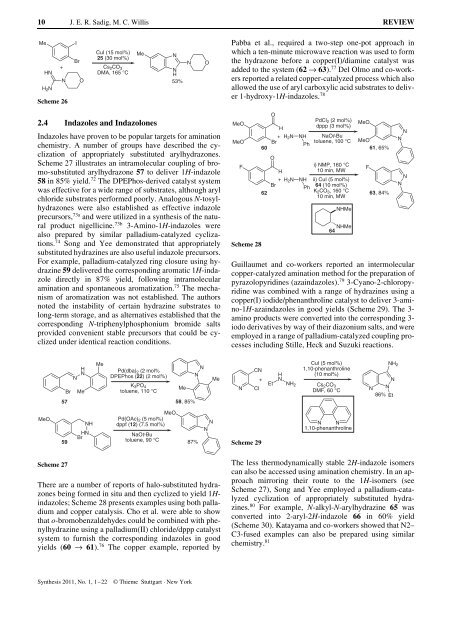
Guillaumet <strong>and</strong> co-workers reported an intermolecular<br />
copper-catalyzed amination method for the preparation of<br />
pyrazolopyridines (azaindazoles). 79 3-Cyano-2-chloropyridine<br />
was combined with a range of hydrazines using a<br />
copper(I) iodide/phenanthroline catalyst to deliver 3-amino-1H-azaindazoles<br />
in good yields (Scheme 29). The 3amino<br />
products were converted into the corresponding 3iodo<br />
derivatives by way of their diazonium salts, <strong>and</strong> were<br />
employed in a range of palladium-catalyzed coupling processes<br />
including Stille, Heck <strong>and</strong> Suzuki reactions.<br />
N<br />
CN<br />
Cl<br />
Scheme 29<br />
O<br />
60<br />
O<br />
+<br />
62<br />
H<br />
+ H2N<br />
Br<br />
H<br />
+ H2N<br />
Br<br />
Et<br />
H<br />
N NH2<br />
NH<br />
Ph<br />
NH<br />
Ph<br />
PdCl 2 (2 mol%)<br />
dppp (3 mol%) MeO<br />
NaOt-Bu<br />
toluene, 100 °C<br />
i) NMP, 160 °C<br />
10 min, MW<br />
ii) CuI (5 mol%)<br />
64 (10 mol%)<br />
K2CO 3, 160 °C<br />
10 min, MW<br />
NHMe<br />
NHMe<br />
MeO<br />
61, 65%<br />
N<br />
N<br />
N<br />
N<br />
The less thermodynamically stable 2H-indazole isomers<br />
can also be accessed using amination chemistry. In an approach<br />
mirroring their route to the 1H-isomers (see<br />
Scheme 27), Song <strong>and</strong> Yee employed a palladium-catalyzed<br />
cyclization of appropriately substituted hydrazines.<br />
80 For example, N-alkyl-N-arylhydrazine 65 was<br />
converted into 2-aryl-2H-indazole 66 in 60% yield<br />
(Scheme 30). Katayama <strong>and</strong> co-workers showed that N2–<br />
C3-fused examples can also be prepared using similar<br />
chemistry. 81<br />
64<br />
CuI (5 mol%)<br />
1,10-phenanthroline<br />
(10 mol%)<br />
Cs 2CO 3<br />
DMF, 60 °C<br />
N N<br />
1,10-phenanthroline<br />
F<br />
63, 84%<br />
NH 2<br />
N<br />
N N<br />
86% Et