Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
REVIEW <strong>Palladium</strong>- <strong>and</strong> <strong>Copper</strong>-<strong>Catalyzed</strong> Heterocycle Synthesis 19<br />
F<br />
H<br />
N<br />
I<br />
O<br />
Me<br />
Na2S<br />
CuI (10 mol%)<br />
DMF, 80 °C<br />
then HCl, r.t. F<br />
N<br />
S<br />
75%<br />
.9H2O<br />
+<br />
Scheme 62<br />
o-halothioacetanilides <strong>and</strong> o-halothiobenzanilides, respectively.<br />
127 Castillón <strong>and</strong> co-workers extended this<br />
methodology to a more efficient palladium-catalyzed cyclization<br />
of o-bromothioamides (Scheme 63). 128 It was<br />
also shown that cyclization of o-bromothioureas, prepared<br />
from o-bromophenylisothiocyanates <strong>and</strong> amines, afforded<br />
2-aminobenzothiazoles under similar reaction conditions<br />
(122 → 123).<br />
H<br />
N<br />
S<br />
Br<br />
122<br />
Scheme 63<br />
Batey <strong>and</strong> co-workers reported a comparison of the copper-<br />
<strong>and</strong> palladium-catalyzed syntheses of 2-aminobenzothiazoles<br />
based on this same cyclization of obromothioureas.<br />
129 The use of copper catalysis generally<br />
led to higher yields <strong>and</strong> conversions. Both metals were<br />
also shown to catalyze an example of a one-pot thiourea<br />
formation–cyclization reaction in the same excellent<br />
yield. Batey’s research group also demonstrated that cyclization<br />
of o-bromothioamides under the same copper(I)<br />
iodide/1,10-phenanthroline catalyst system afforded benzothiazoles<br />
in excellent yields; 115 a representative example<br />
is shown in Scheme 64. Jiang <strong>and</strong> Ma reported a<br />
related copper-catalyzed cyclization employing oxazolidin-2-one<br />
as the lig<strong>and</strong>; a number of aryl chloride substrates<br />
were included in their study, <strong>and</strong> effectively<br />
converted into benzothiazoles. 117 Pan <strong>and</strong> co-workers reported<br />
a related preparation of 2-aminobenzothiazoles using<br />
a copper(I) iodide/oxazoline catalyst system. 130<br />
Scheme 64<br />
H<br />
N Ph<br />
+<br />
O<br />
Br<br />
Me Me<br />
Me<br />
H<br />
N NMe 2<br />
S<br />
Br<br />
H<br />
N<br />
S<br />
Br<br />
K 2S<br />
CuI (10 mol%)<br />
DMF, 140 °C<br />
then HCl, r.t.<br />
Pd2(dba)3 (5 mol%)<br />
JohnPhos (106)<br />
(5.5 mol%)<br />
Cs2CO 3<br />
dioxane, 80 °C<br />
Pd 2(dba) 3 (5 mol%)<br />
P(t-Bu)3 (5.5 mol%)<br />
OMe<br />
Cs2CO 3<br />
dioxane, 80 °C<br />
CuI (5 mol%)<br />
1,10-phenanthroline<br />
(10 mol%)<br />
Cs 2CO3, reflux<br />
88%<br />
N<br />
S<br />
Me<br />
Ph<br />
N Me<br />
Me<br />
S Me<br />
88%<br />
N<br />
S<br />
123, 92%<br />
N<br />
S<br />
93%<br />
NMe 2<br />
OMe<br />
The Wu research group described the synthesis of 2-aminobenzothiazoles<br />
by the reaction of o-iodobenzamines<br />
with isothiocyanates using a copper(I) iodide/phenanthroline<br />
catalyst system (Scheme 65). 131 The method was useful<br />
as it eliminated the need to generate an ohalobenzothiourea<br />
cyclization precursor in a separate<br />
step, with the addition/carbon–sulfur coupling reaction<br />
occurring in one pot. The authors exploited the method in<br />
the preparation of an 18-membered library.<br />
F<br />
Scheme 65<br />
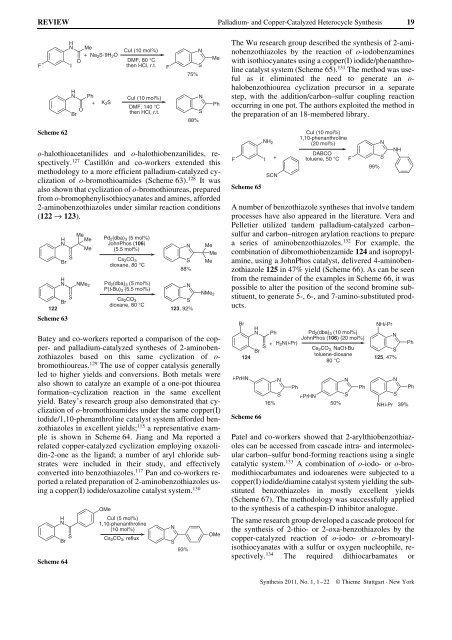
A number of benzothiazole syntheses that involve t<strong>and</strong>em<br />
processes have also appeared in the literature. Vera <strong>and</strong><br />
Pelletier utilized t<strong>and</strong>em palladium-catalyzed carbon–<br />
sulfur <strong>and</strong> carbon–nitrogen arylation reactions to prepare<br />
a series of aminobenzothiazoles. 132 For example, the<br />
combination of dibromothiobenzamide 124 <strong>and</strong> isopropylamine,<br />
using a JohnPhos catalyst, delivered 4-aminobenzothiazole<br />
125 in 47% yield (Scheme 66). As can be seen<br />
from the remainder of the examples in Scheme 66, it was<br />
possible to alter the position of the second bromine substituent,<br />
to generate 5-, 6-, <strong>and</strong> 7-amino-substituted products.<br />
Scheme 66<br />
NH 2<br />
I<br />
+<br />
SCN<br />
CuI (10 mol%)<br />
1,10-phenanthroline<br />
(20 mol%)<br />
DABCO<br />
toluene, 50 °C<br />
99%<br />
Br NHi-Pr<br />
124<br />
i-PrHN<br />
H<br />
N Ph<br />
+<br />
H2N(i-Pr)<br />
S<br />
Br<br />
16%<br />
N<br />
S<br />
Ph<br />
Pd 2(dba) 3 (10 mol%)<br />
JohnPhos (106) (20 mol%)<br />
i-PrHN<br />
Patel <strong>and</strong> co-workers showed that 2-arylthiobenzothiazoles<br />
can be accessed from cascade intra- <strong>and</strong> intermolecular<br />
carbon–sulfur bond-forming reactions using a single<br />
catalytic system. 133 A combination of o-iodo- or o-bromodithiocarbamates<br />
<strong>and</strong> iodoarenes were subjected to a<br />
copper(I) iodide/diamine catalyst system yielding the substituted<br />
benzothiazoles in mostly excellent yields<br />
(Scheme 67). The methodology was successfully applied<br />
to the synthesis of a cathespin-D inhibitor analogue.<br />
The same research group developed a cascade protocol for<br />
the synthesis of 2-thio- or 2-oxa-benzothiazoles by the<br />
copper-catalyzed reaction of o-iodo- or o-bromoarylisothiocyanates<br />
with a sulfur or oxygen nucleophile, respectively.<br />
134 The required dithiocarbamates or<br />
Synthesis 2011, No. 1, 1–22 © Thieme Stuttgart · New York<br />
F<br />
Cs 2CO 3, NaOt-Bu<br />
toluene-dioxane<br />
80 °C<br />
50%<br />
N<br />
S<br />
Ph<br />
N<br />
S<br />
NH<br />
N<br />
S<br />
125, 47%<br />
N<br />
S<br />
NHi-Pr 39%<br />
Ph<br />
Ph