Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
REVIEW <strong>Palladium</strong>- <strong>and</strong> <strong>Copper</strong>-<strong>Catalyzed</strong> Heterocycle Synthesis 11<br />
N Ph<br />
MeO<br />
Pd(OAc) 2 (5 mol%)<br />
MeO<br />
dppf (12) (7.5 mol%)<br />
NH2 Br<br />
NaOt-Bu<br />
toluene, 90 °C<br />
65<br />
Scheme 30<br />
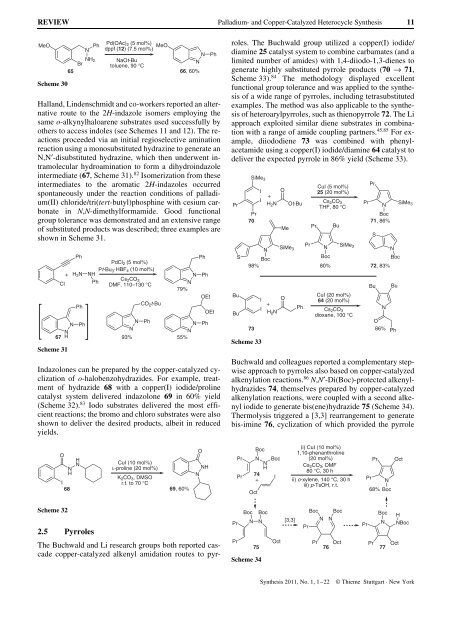
Hall<strong>and</strong>, Lindenschmidt <strong>and</strong> co-workers reported an alternative<br />
route to the 2H-indazole isomers employing the<br />
same o-alkynylhaloarene substrates used successfully by<br />
others to access indoles (see Schemes 11 <strong>and</strong> 12). The reactions<br />
proceeded via an initial regioselective amination<br />
reaction using a monosubstituted hydrazine to generate an<br />
N,N¢-disubstituted hydrazine, which then underwent intramolecular<br />
hydroamination to form a dihydroindazole<br />
intermediate (67, Scheme 31). 82 Isomerization from these<br />
intermediates to the aromatic 2H-indazoles occurred<br />
spontaneously under the reaction conditions of palladium(II)<br />
chloride/tri(tert-butyl)phosphine with cesium carbonate<br />
in N,N-dimethylformamide. Good functional<br />
group tolerance was demonstrated <strong>and</strong> an extensive range<br />
of substituted products was described; three examples are<br />
shown in Scheme 31.<br />
67<br />
Scheme 31<br />
Indazolones can be prepared by the copper-catalyzed cyclization<br />
of o-halobenzohydrazides. For example, treatment<br />
of hydrazide 68 with a copper(I) iodide/proline<br />
catalyst system delivered indazolone 69 in 60% yield<br />
(Scheme 32). 83 Iodo substrates delivered the most efficient<br />
reactions; the bromo <strong>and</strong> chloro substrates were also<br />
shown to deliver the desired products, albeit in reduced<br />
yields.<br />
Scheme 32<br />
Ph<br />
+ H2N<br />
Cl<br />
O<br />
I<br />
Ph<br />
N Ph<br />
N<br />
H<br />
N<br />
H<br />
H<br />
N<br />
2.5 Pyrroles<br />
PdCl2 (5 mol%)<br />
Pt-Bu3 HBF4 (10 mol%)<br />
NH<br />
Cs2CO<br />
Ph<br />
3<br />
DMF, 110–130 °C<br />
.<br />
93%<br />
CO2t-Bu<br />
N Ph<br />
N<br />
CuI (10 mol%)<br />
L-proline (20 mol%)<br />
K 2CO 3, DMSO<br />
r.t. to 70 °C<br />
68 69, 60%<br />
N Ph<br />
N<br />
66, 60%<br />
N Ph<br />
N<br />
79%<br />
OEt<br />
The Buchwald <strong>and</strong> Li research groups both reported cascade<br />
copper-catalyzed alkenyl amidation routes to pyr-<br />
Ph<br />
N Ph<br />
N<br />
55%<br />
O<br />
OEt<br />
NH<br />
N<br />
roles. The Buchwald group utilized a copper(I) iodide/<br />
diamine 25 catalyst system to combine carbamates (<strong>and</strong> a<br />
limited number of amides) with 1,4-diiodo-1,3-dienes to<br />
generate highly substituted pyrrole products (70 → 71,<br />
Scheme 33). 84 The methodology displayed excellent<br />
functional group tolerance <strong>and</strong> was applied to the synthesis<br />
of a wide range of pyrroles, including tetrasubstituted<br />
examples. The method was also applicable to the synthesis<br />
of heteroarylpyrroles, such as thienopyrrole 72. The Li<br />
approach exploited similar diene substrates in combination<br />
with a range of amide coupling partners. 45,85 For example,<br />
diiododiene 73 was combined with phenylacetamide<br />
using a copper(I) iodide/diamine 64 catalyst to<br />
deliver the expected pyrrole in 86% yield (Scheme 33).<br />
Pr<br />
S<br />
Bu<br />
Bu<br />
SiMe3<br />
Pr<br />
70<br />
73<br />
Scheme 33<br />
I O<br />
I<br />
+<br />
H2N Ot-Bu<br />
Me<br />
Pr<br />
CuI (5 mol%)<br />
25 (20 mol%)<br />
Cs 2CO 3<br />
THF, 80 °C<br />
CuI (20 mol%)<br />
64 (20 mol%)<br />
Cs 2CO 3<br />
dioxane, 100 °C<br />
Pr<br />
N<br />
Boc<br />
71, 86%<br />
N<br />
SiMe3<br />
Pr<br />
N<br />
SiMe3<br />
N<br />
Boc<br />
Boc<br />
Boc<br />
98% 80%<br />
72, 83%<br />
I O<br />
+<br />
I<br />
H2N Ph<br />
Buchwald <strong>and</strong> colleagues reported a complementary stepwise<br />
approach to pyrroles also based on copper-catalyzed<br />
alkenylation reactions. 86 N,N¢-Di(Boc)-protected alkenylhydrazides<br />
74, themselves prepared by copper-catalyzed<br />
alkenylation reactions, were coupled with a second alkenyl<br />
iodide to generate bis(ene)hydrazide 75 (Scheme 34).<br />
Thermolysis triggered a [3,3] rearrangement to generate<br />
bis-imine 76, cyclization of which provided the pyrrole<br />
Pr<br />
Pr<br />
Pr<br />
Boc<br />
Oct<br />
N<br />
Boc<br />
N<br />
N<br />
H<br />
Boc<br />
74<br />
+<br />
Boc<br />
N<br />
Pr Oct<br />
I<br />
[3,3]<br />
Bu<br />
(i) CuI (10 mol%)<br />
1,10-phenanthroline<br />
(20 mol%)<br />
Cs2CO3, DMF<br />
80 °C, 30 h<br />
ii) o-xylene, 140 °C, 30 h<br />
iii) p-TsOH, r.t.<br />
Boc Boc<br />
N N<br />
Pr<br />
Pr Oct<br />
Synthesis 2011, No. 1, 1–22 © Thieme Stuttgart · New York<br />
Pr<br />
S<br />
Bu<br />
N<br />
Bu<br />
O<br />
86% Ph<br />
75 76 77<br />
Scheme 34<br />
Pr<br />
Pr<br />
Pr Oct<br />
N<br />
68% Boc<br />
Boc<br />
N<br />
Pr Oct<br />
SiMe3<br />
H<br />
NBoc