Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
REVIEW <strong>Palladium</strong>- <strong>and</strong> <strong>Copper</strong>-<strong>Catalyzed</strong> Heterocycle Synthesis 17<br />
cles in good yields from either the o-iodo, o-bromo- or ochlorobenzanilides.<br />
116 Similar cyclizations have also been<br />
reported by Jiang <strong>and</strong> Ma 117 <strong>and</strong> Kantam <strong>and</strong> co-workers.<br />
118<br />
H<br />
N<br />
Br<br />
N<br />
Scheme 54<br />
In 2009 Sun <strong>and</strong> co-workers extended this type of cyclization<br />
to the synthesis of 2-substituted oxazolopyridines. 119<br />
As shown in Scheme 55, a copper(I) iodide/diamine or<br />
copper(I) iodide/phenanthroline catalyst system proved to<br />
be optimal, yielding the desired heterocycles from the cyclization<br />
of o-chloro- or o-bromopyridylamides, respectively.<br />
Me<br />
N<br />
Scheme 55<br />
CuI (5 mol%)<br />
1,10-phenanthroline<br />
(10 mol%)<br />
O OMe Cs 2CO 3<br />
DME, relfux<br />
O Ph<br />
89%<br />
N N<br />
O<br />
90%<br />
Cbz<br />
25<br />
H<br />
N<br />
CuI (5 mol%)<br />
(10 mol%)<br />
N<br />
O<br />
Cl<br />
K2CO3 toluene, reflux<br />
N O<br />
91%<br />
N<br />
H<br />
N<br />
O<br />
Br<br />
Ph<br />
Cl<br />
CuI (5 mol%)<br />
1,10-phenanthroline<br />
(10 mol%)<br />
Cs2CO3<br />
THF, reflux<br />
MeO<br />
N<br />
The Batey research group developed a further synthesis of<br />
benzoxazoles involving intramolecular carbon–oxygen<br />
bond formation. The substrates were again o-halobenzanilides;<br />
however, these were formed in situ from the reaction<br />
of o-bromoanilines <strong>and</strong> acyl chlorides. 70 A copper(I)<br />
iodide/1,10-phenanthroline combination was found to be<br />
the optimal catalyst system for this one-pot strategy. In<br />
addition, the use of microwave irradiation gave substantially<br />
better results than conventional heating. A library of<br />
24 benzoxazoles was prepared, examples of which are<br />
shown in Scheme 56.<br />
A t<strong>and</strong>em approach to benzoxazoles was reported by<br />
Glorius <strong>and</strong> Altenhoff, in which reaction of o-dihalobenzenes<br />
with amides underwent copper(I) iodide/diamine<br />
catalyzed carbon–nitrogen followed by carbon–oxygen<br />
cross-couplings to yield the desired heterocycles. 120 Examples<br />
of diiodo-, dibromo- <strong>and</strong> mixed dihalobenzenes,<br />
as well as dihalopyridines (Br <strong>and</strong> Cl) were successfully<br />
coupled to benzamide affording 2-phenylbenzoxazoles<br />
(Scheme 57). The dibromobenzene substrate was utilized<br />
to demonstrate variation of the amide partner, giving aryl,<br />
Me<br />
N<br />
O<br />
99%<br />
N<br />
O<br />
90%<br />
97%<br />
N<br />
O<br />
Ph<br />
Ph<br />
Cl<br />
Me<br />
S<br />
O<br />
+<br />
Scheme 56<br />
alkyl, vinyl <strong>and</strong> heterocyclic 2-substituted benzoxazoles.<br />
The use of o-bromochlorobenzenes allowed the regioselective<br />
synthesis of substituted benzoxazoles, with the reaction<br />
proceeding via initial amidation at the aryl bromide<br />
position. Batey <strong>and</strong> co-workers had previously reported<br />
on investigations of related processes, but had not<br />
achieved an efficient system. 70<br />
Scheme 57<br />
Cl<br />
O<br />
NH 2<br />
Br<br />
N<br />
3.3 Isocoumarins<br />
CuI (10 mol%)<br />
1,10-phenanthroline<br />
(20 mol%)<br />
Cs 2CO3, MeCN<br />
210 °C, 15 min, MW<br />
O<br />
91%<br />
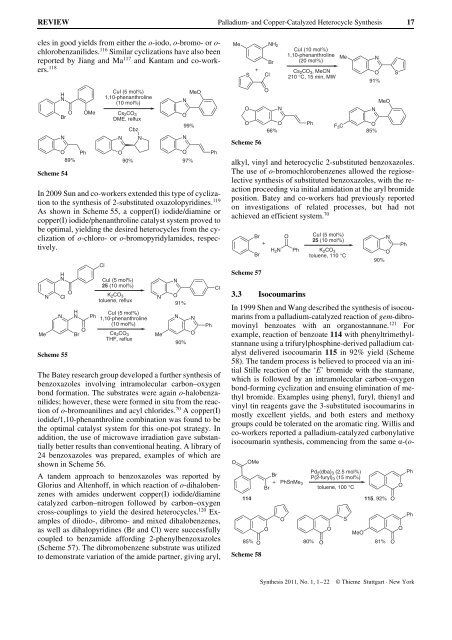
In 1999 Shen <strong>and</strong> Wang described the synthesis of isocoumarins<br />
from a palladium-catalyzed reaction of gem-dibromovinyl<br />
benzoates with an organostannane. 121 For<br />
example, reaction of benzoate 114 with phenyltrimethylstannane<br />
using a trifurylphosphine-derived palladium catalyst<br />
delivered isocoumarin 115 in 92% yield (Scheme<br />
58). The t<strong>and</strong>em process is believed to proceed via an initial<br />
Stille reaction of the ‘E’ bromide with the stannane,<br />
which is followed by an intramolecular carbon–oxygen<br />
bond-forming cyclization <strong>and</strong> ensuing elimination of methyl<br />
bromide. Examples using phenyl, furyl, thienyl <strong>and</strong><br />
vinyl tin reagents gave the 3-substituted isocoumarins in<br />
mostly excellent yields, <strong>and</strong> both esters <strong>and</strong> methoxy<br />
groups could be tolerated on the aromatic ring. Willis <strong>and</strong><br />
co-workers reported a palladium-catalyzed carbonylative<br />
isocoumarin synthesis, commencing from the same a-(o-<br />
Synthesis 2011, No. 1, 1–22 © Thieme Stuttgart · New York<br />
Me<br />
O O Ph<br />
F3C<br />
O<br />
66%<br />
85%<br />
Br<br />
+<br />
Br<br />
O OMe<br />
114<br />
85% O<br />
Scheme 58<br />
O<br />
H 2N Ph<br />
Br<br />
Br<br />
+<br />
PhSnMe3 O<br />
O<br />
CuI (5 mol%)<br />
25 (10 mol%)<br />
K2CO 3<br />
toluene, 110 °C<br />
80%<br />
Pd2(dba)3 (2.5 mol%)<br />
P(2-furyl)3 (15 mol%)<br />
toluene, 100 °C<br />
O<br />
O<br />
S<br />
MeO<br />
N<br />
MeO<br />
N<br />
N<br />
O<br />
90%<br />
115, 92%<br />
81%<br />
O<br />
O<br />
S<br />
Ph<br />
O<br />
O<br />
Ph<br />
Ph