Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
REVIEW <strong>Palladium</strong>- <strong>and</strong> <strong>Copper</strong>-<strong>Catalyzed</strong> Heterocycle Synthesis 9<br />
MeO<br />
Scheme 21<br />
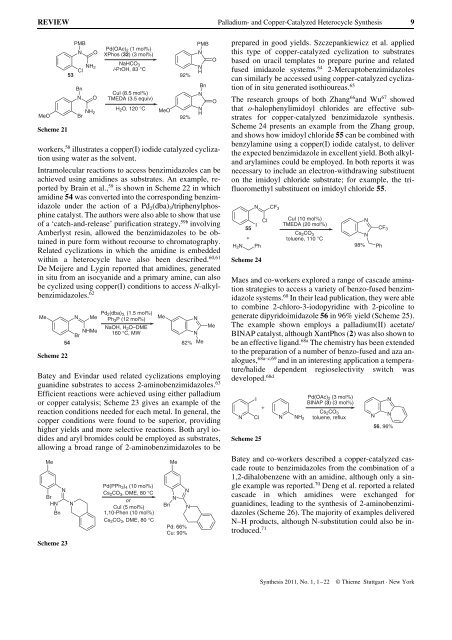
workers, 58 illustrates a copper(I) iodide catalyzed cyclization<br />
using water as the solvent.<br />
Intramolecular reactions to access benzimidazoles can be<br />
achieved using amidines as substrates. An example, reported<br />
by Brain et al., 59 is shown in Scheme 22 in which<br />
amidine 54 was converted into the corresponding benzimidazole<br />
under the action of a Pd2(dba) 3/triphenylphosphine<br />
catalyst. The authors were also able to show that use<br />
of a ‘catch-<strong>and</strong>-release’ purification strategy, 59b involving<br />
Amberlyst resin, allowed the benzimidazoles to be obtained<br />
in pure form without recourse to chromatography.<br />
Related cyclizations in which the amidine is embedded<br />
within a heterocycle have also been described. 60,61<br />
De Meijere <strong>and</strong> Lygin reported that amidines, generated<br />
in situ from an isocyanide <strong>and</strong> a primary amine, can also<br />
be cyclized using copper(I) conditions to access N-alkylbenzimidazoles.<br />
62<br />
Me<br />
53<br />
54<br />
Scheme 22<br />
PMB PMB<br />
Pd(OAc) 2 (1 mol%)<br />
N O XPhos (32) (3 mol%)<br />
N<br />
NH2<br />
Cl<br />
Bn<br />
N<br />
O<br />
NH2 Br<br />
NaHCO3<br />
i-PrOH, 83 °C<br />
CuI (8.5 mol%)<br />
TMEDA (3.5 equiv)<br />
H 2O, 120 °C<br />
MeO<br />
92%<br />
92%<br />
Batey <strong>and</strong> Evindar used related cyclizations employing<br />
guanidine substrates to access 2-aminobenzimidazoles. 63<br />
Efficient reactions were achieved using either palladium<br />
or copper catalysis; Scheme 23 gives an example of the<br />
reaction conditions needed for each metal. In general, the<br />
copper conditions were found to be superior, providing<br />
higher yields <strong>and</strong> more selective reactions. Both aryl iodides<br />
<strong>and</strong> aryl bromides could be employed as substrates,<br />
allowing a broad range of 2-aminobenzimidazoles to be<br />
Me<br />
Br<br />
HN<br />
Bn<br />
N<br />
Scheme 23<br />
N<br />
N Me<br />
NHMe<br />
Br<br />
Pd2(dba)3, (1.5 mol%)<br />
Ph3P (12 mol%)<br />
NaOH, H 2O–DME<br />
160 °C, MW<br />
Pd(PPh3)4 (10 mol%)<br />
Cs2CO3, DME, 80 °C<br />
or<br />
CuI (5 mol%)<br />
1,10-Phen (10 mol%)<br />
Cs2CO3, DME, 80 °C<br />
Me<br />
Me<br />
82%<br />
N<br />
N<br />
Bn N<br />
Pd: 66%<br />
Cu: 90%<br />
N<br />
N<br />
N<br />
H<br />
Bn<br />
N<br />
N<br />
H<br />
Me<br />
O<br />
O<br />
Me<br />
prepared in good yields. Szczepankiewicz et al. applied<br />
this type of copper-catalyzed cyclization to substrates<br />
based on uracil templates to prepare purine <strong>and</strong> related<br />
fused imidazole systems. 64 2-Mercaptobenzimidazoles<br />
can similarly be accessed using copper-catalyzed cyclization<br />
of in situ generated isothioureas. 65<br />
The research groups of both Zhang66<strong>and</strong> Wu67 showed<br />
that o-halophenylimidoyl chlorides are effective substrates<br />
for copper-catalyzed benzimidazole synthesis.<br />
Scheme 24 presents an example from the Zhang group,<br />
<strong>and</strong> shows how imidoyl chloride 55 can be combined with<br />
benzylamine using a copper(I) iodide catalyst, to deliver<br />
the expected benzimidazole in excellent yield. Both alkyl<strong>and</strong><br />
arylamines could be employed. In both reports it was<br />
necessary to include an electron-withdrawing substituent<br />
on the imidoyl chloride substrate; for example, the trifluoromethyl<br />
substituent on imidoyl chloride 55.<br />
N<br />
Scheme 24<br />
Maes <strong>and</strong> co-workers explored a range of cascade amination<br />
strategies to access a variety of benzo-fused benzimidazole<br />
systems. 68 In their lead publication, they were able<br />
to combine 2-chloro-3-iodopyridine with 2-picoline to<br />
generate dipyridoimidazole 56 in 96% yield (Scheme 25).<br />
The example shown employs a palladium(II) acetate/<br />
BINAP catalyst, although XantPhos (2) was also shown to<br />
be an effective lig<strong>and</strong>. 68a The chemistry has been extended<br />
to the preparation of a number of benzo-fused <strong>and</strong> aza analogues,<br />
68a–c,69 <strong>and</strong> in an interesting application a temperature/halide<br />
dependent regioselectivity switch was<br />
developed. 68d<br />
Scheme 25<br />
I<br />
H2N Ph<br />
N<br />
55<br />
+<br />
I<br />
Cl<br />
+<br />
CF3<br />
Cl N NH 2<br />
CuI (10 mol%)<br />
TMEDA (20 mol%)<br />
Cs 2CO 3<br />
toluene, 110 °C<br />
Pd(OAc) 2 (3 mol%)<br />
BINAP (3) (3 mol%)<br />
Cs2CO3<br />
toluene, reflux<br />
98%<br />
Batey <strong>and</strong> co-workers described a copper-catalyzed cascade<br />
route to benzimidazoles from the combination of a<br />
1,2-dihalobenzene with an amidine, although only a single<br />
example was reported. 70 Deng et al. reported a related<br />
cascade in which amidines were exchanged for<br />
guanidines, leading to the synthesis of 2-aminobenzimidazoles<br />
(Scheme 26). The majority of examples delivered<br />
N–H products, although N-substitution could also be introduced.<br />
71<br />
Synthesis 2011, No. 1, 1–22 © Thieme Stuttgart · New York<br />
N<br />
N<br />
N<br />
CF3<br />
Ph<br />
N<br />
N<br />
56, 96%